AKR1C3 expression in T acute lymphoblastic leukemia/lymphoma for clinical use as a biomarker
- PMID: 35388063
- PMCID: PMC8986791
- DOI: 10.1038/s41598-022-09697-6
AKR1C3 expression in T acute lymphoblastic leukemia/lymphoma for clinical use as a biomarker
Abstract
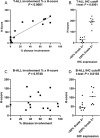
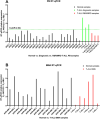
To investigate aldo-keto reductase 1C3 (AKR1C3) expression in T and B acute lymphoblastic leukemia/lymphoma (ALL) patients. Three commercial antibodies were evaluated for AKR1C3 immunohistochemistry (IHC) staining performance: Polyclonal Thermofisher scientific (Clone#PA523667), rabbit monoclonal Abcam [EPR16726] (ab209899) and Sigma/Millipore anti-AKR1C3 antibody, mouse monoclonal, clone NP6.G6.A6, purified from hybridoma cell culture. Initial optimization was performed on cell line controls: HCT116 (negative control); genetically modified cell line HCT116 with AKR1C3 overexpression; Nalm and TF1 cell lines. Twenty normal bone marrows from archival B and T-ALL patient samples were subsequently examined. AKR1C3 expression levels in these samples were evaluated by immunohistochemistry, Protein Wes and quantitative RT-PCR. Sigma/Millipore Anti-AKR1C3 antibody (mouse monoclonal, clone NP6.G6.A6) showed higher specificity compared to rabbit polyclonal antibody by immunohistochemistry. H-score was used to quantify percent of nuclear immunoreactivity for AKR1C3 with varying disease involvement. T-ALL samples had a higher H-score (172-190) compared to B-ALL cases (H-score, 30-160). The AKR1C3 expression in peripheral blood by Protein Wes and RT-qPCR showed concordance in relapsed/refractory and/or minimal residual T-ALL cases. Sigma/Millipore Anti-AKR1C3 antibody and mouse monoclonal, clone NP6.G6.A6 can be used to aid in AKR1C expression of T-ALL and in cases of relapsed/refractory and/or minimal residual disease.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

