ZOOMICS: Comparative Metabolomics of Red Blood Cells From Guinea Pigs, Humans, and Non-human Primates During Refrigerated Storage for Up to 42 Days
- PMID: 35388289
- PMCID: PMC8977988
- DOI: 10.3389/fphys.2022.845347
ZOOMICS: Comparative Metabolomics of Red Blood Cells From Guinea Pigs, Humans, and Non-human Primates During Refrigerated Storage for Up to 42 Days
Abstract
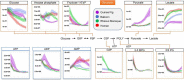
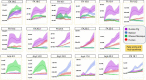
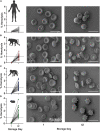
Unlike other rodents, guinea pigs (Cavia porcellus) have evolutionarily lost their capacity to synthesize vitamin C (ascorbate) de novo and, like several non-human primates and humans, rely on dietary intake and glutathione-dependent recycling to cope with oxidant stress. This is particularly relevant in red blood cell physiology, and especially when modeling blood storage, which exacerbates erythrocyte oxidant stress. Herein we provide a comprehensive metabolomics analysis of fresh and stored guinea pig red blood cell concentrates (n = 20), with weekly sampling from storage day 0 through 42. Results were compared to previously published ZOOMICS studies on red blood cells from three additional species with genetic loss of L-gulonolactone oxidase function, including humans (n = 21), olive baboons (n = 20), and rhesus macaques (n = 20). While metabolic trends were comparable across all species, guinea pig red blood cells demonstrated accelerated alterations of the metabolic markers of the storage lesion that are consistent with oxidative stress. Compared to the other species, guinea pig red blood cells showed aberrant glycolysis, pentose phosphate pathway end product metabolites, purine breakdown products, methylation, glutaminolysis, and markers of membrane lipid remodeling. Consistently, guinea pig red blood cells demonstrated higher end storage hemolysis, and scanning electron microscopy confirmed a higher degree of morphological alterations of their red blood cells, as compared to the other species. Despite a genetic inability to produce ascorbate that is common to the species evaluated, guinea pig red blood cells demonstrate accelerated oxidant stress under standard storage conditions. These data may offer relevant insights into the basal and cold storage metabolism of red blood cells from species that cannot synthesize endogenous ascorbate.
Keywords: ascorbate; comparative biology; erythrocyte; hemolysis; metabolomics; rodent.
Copyright © 2022 Bertolone, Shin, Baek, Gao, Spitalnik, Buehler and D’Alessandro.
Conflict of interest statement
AD’A is a founder of Omix Technologies Inc. AD’A is also a consultant for Altis Biosciences LLC., Rubius Inc., and Forma Inc. AD’A and SS are both consultants for Hemanext Inc. SS is also a consultant for Tioma, Inc., TCIP, Inc., and the Executive Director of the Worldwide Initiative for Rh Disease Eradication (WIRhE). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
ZOOMICS : comparative metabolomics of red blood cells from dogs, cows, horses and donkeys during refrigerated storage for up to 42 days.Blood Transfus. 2023 Jul;21(4):314-326. doi: 10.2450/2022.0118-22. Epub 2022 Jul 25. Blood Transfus. 2023. PMID: 35969134 Free PMC article.
-
ZOOMICS: Comparative Metabolomics of Red Blood Cells From Old World Monkeys and Humans.Front Physiol. 2020 Oct 23;11:593841. doi: 10.3389/fphys.2020.593841. eCollection 2020. Front Physiol. 2020. PMID: 33192610 Free PMC article.
-
Red blood cell metabolism in Rhesus macaques and humans: comparative biology of blood storage.Haematologica. 2020 Aug;105(8):2174-2186. doi: 10.3324/haematol.2019.229930. Epub 2019 Nov 7. Haematologica. 2020. PMID: 31699790 Free PMC article.
-
Final report of the safety assessment of L-Ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate as used in cosmetics.Int J Toxicol. 2005;24 Suppl 2:51-111. doi: 10.1080/10915810590953851. Int J Toxicol. 2005. PMID: 16154915 Review.
-
Birth and Neonatal Transition in the Guinea Pig: Experimental Approaches to Prevent Preterm Birth and Protect the Premature Fetus.Front Physiol. 2018 Dec 11;9:1802. doi: 10.3389/fphys.2018.01802. eCollection 2018. Front Physiol. 2018. PMID: 30618814 Free PMC article. Review.
Cited by
-
ZOOMICS : comparative metabolomics of red blood cells from dogs, cows, horses and donkeys during refrigerated storage for up to 42 days.Blood Transfus. 2023 Jul;21(4):314-326. doi: 10.2450/2022.0118-22. Epub 2022 Jul 25. Blood Transfus. 2023. PMID: 35969134 Free PMC article.
-
Omics Technologies in Veterinary Medicine: Literature Review and Perspectives in Transfusion Medicine.Transfus Med Hemother. 2023 May 25;50(3):198-207. doi: 10.1159/000530870. eCollection 2023 Jun. Transfus Med Hemother. 2023. PMID: 37408648 Free PMC article. Review.
-
Impact of leukoreduction on the metabolome of ovine packed red blood cells during refrigerated storage.Blood Transfus. 2025 Jul-Aug;23(4):304-317. doi: 10.2450/BloodTransfus.830. Epub 2025 Feb 6. Blood Transfus. 2025. PMID: 39950860 Free PMC article.
-
Safety and efficacy of human polymerized hemoglobin on guinea pig resuscitation from hemorrhagic shock.Sci Rep. 2022 Nov 28;12(1):20480. doi: 10.1038/s41598-022-23926-y. Sci Rep. 2022. PMID: 36443351 Free PMC article.
-
Ultraviolet light and riboflavin accelerates red blood cell dysfunction in vitro and in a guinea pig transfusion model.Blood Transfus. 2024 Jul 15;22(4):316-327. doi: 10.2450/BloodTransfus.718. Blood Transfus. 2024. PMID: 38814883 Free PMC article.
References
-
- Aktan B., Taysi S., Gumustekin K., Bakan N., Sutbeyaz Y. (2003). Evaluation of oxidative stress in erythrocytes of guinea pigs with experimental otitis media and effusion. Ann. Clin. Lab. Sci. 33 232–236. - PubMed
-
- Baek J. H., D’Agnillo F., Vallelian F., Pereira C. P., Williams M. C., Jia Y., et al. (2012). Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J. Clin. Invest. 122, 1444–1458. 10.1172/JCI59770 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

