Diffusion tractography predicts propagated high-frequency activity during epileptic spasms
- PMID: 35388455
- PMCID: PMC9283246
- DOI: 10.1111/epi.17251
Diffusion tractography predicts propagated high-frequency activity during epileptic spasms
Abstract
Objective: To determine the structural networks that constrain propagation of ictal oscillations during epileptic spasm events, and compare the observed propagation patterns across patients with successful or unsuccessful surgical outcomes.
Methods: Subdural electrode recordings of 18 young patients (age 1-11 years) were analyzed during epileptic spasm events to determine ictal networks and quantify the amplitude and onset time of ictal oscillations across the cortical surface. Corresponding structural networks were generated with diffusion magnetic resonance imaging (MRI) tractography by seeding the cortical region associated with the earliest average oscillation onset time, and white matter pathways connecting active electrode regions within the ictal network were isolated. Properties of this structural network were used to predict oscillation onset times and amplitudes, and this relationship was compared across patients who did and did not achieve seizure freedom following resective surgery.
Results: Onset propagation patterns were relatively consistent across each patient's spasm events. An electrode's average ictal oscillation onset latency was most significantly associated with the length of direct corticocortical tracts connecting to the area with the earliest average oscillation onset (p < .001, model R2 = .54). Moreover, patients demonstrating a faster propagation of ictal oscillation signals within the corticocortical network were more likely to have seizure recurrence following resective surgery (p = .039). In addition, ictal oscillation amplitude was associated with connecting tractography length and weighted fractional anisotropy (FA) measures along these pathways (p = .002/.030, model R2 = .31/.25). Characteristics of analogous corticothalamic pathways did not show significant associations with ictal oscillation onset latency or amplitude.
Significance: Spatiotemporal propagation patterns of high-frequency activity in epileptic spasms align with length and FA measures from onset-originating corticocortical pathways. Considering the data in this individualized framework may help inform surgical decision-making and expectations of surgical outcomes.
Keywords: diffusion MRI tractography; high-frequency oscillations; infantile spasms; intracranial electroencephalography; seizure propagation; surgical outcome.
© 2022 International League Against Epilepsy.
Conflict of interest statement
Disclosures
None of the authors has any conflict of interest to disclose. The authors confirm that they have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
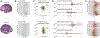



References
-
- Aaberg KM, Gunnes N, Bakken IJ, Lund Søraas C, Berntsen A, Magnus P, et al. Incidence and Prevalence of Childhood Epilepsy: A Nationwide Cohort Study. Pediatrics. 2017; 139(5):e20163908. - PubMed
-
- Wilmshurst JM, Ibekwe RC, O’Callaghan FJK. Epileptic spasms — 175 years on: Trying to teach an old dog new tricks. Seizure. 2017; 44:81–6. - PubMed
-
- Pavone P, Striano P, Falsaperla R, Pavone L, Ruggieri M. Infantile spasms syndrome, West syndrome and related phenotypes: What we know in 2013. Brain Dev. 2014; 36(9):739–51. - PubMed
-
- Widjaja E, Go C, McCoy B, Snead OC. Neurodevelopmental outcome of infantile spasms: A systematic review and meta-analysis. Epilepsy Res. 2015; 109:155–62. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

