From fibre to function: are we accurately representing muscle architecture and performance?
- PMID: 35388613
- PMCID: PMC9540431
- DOI: 10.1111/brv.12856
From fibre to function: are we accurately representing muscle architecture and performance?
Abstract
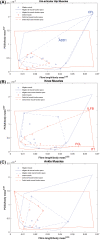
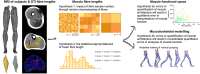
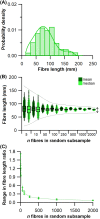
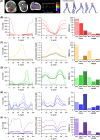
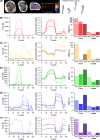
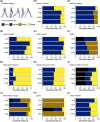
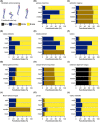
The size and arrangement of fibres play a determinate role in the kinetic and energetic performance of muscles. Extrapolations between fibre architecture and performance underpin our understanding of how muscles function and how they are adapted to power specific motions within and across species. Here we provide a synopsis of how this 'fibre to function' paradigm has been applied to understand muscle design, performance and adaptation in animals. Our review highlights the widespread application of the fibre to function paradigm across a diverse breadth of biological disciplines but also reveals a potential and highly prevalent limitation running through past studies. Specifically, we find that quantification of muscle architectural properties is almost universally based on an extremely small number of fibre measurements. Despite the volume of research into muscle properties, across a diverse breadth of research disciplines, the fundamental assumption that a small proportion of fibre measurements can accurately represent the architectural properties of a muscle has never been quantitatively tested. Subsequently, we use a combination of medical imaging, statistical analysis, and physics-based computer simulation to address this issue for the first time. By combining diffusion tensor imaging (DTI) and deterministic fibre tractography we generated a large number of fibre measurements (>3000) rapidly for individual human lower limb muscles. Through statistical subsampling simulations of these measurements, we demonstrate that analysing a small number of fibres (n < 25) typically used in previous studies may lead to extremely large errors in the characterisation of overall muscle architectural properties such as mean fibre length and physiological cross-sectional area. Through dynamic musculoskeletal simulations of human walking and jumping, we demonstrate that recovered errors in fibre architecture characterisation have significant implications for quantitative predictions of in-vivo dynamics and muscle fibre function within a species. Furthermore, by applying data-subsampling simulations to comparisons of muscle function in humans and chimpanzees, we demonstrate that error magnitudes significantly impact both qualitative and quantitative assessment of muscle specialisation, potentially generating highly erroneous conclusions about the absolute and relative adaption of muscles across species and evolutionary transitions. Our findings have profound implications for how a broad diversity of research fields quantify muscle architecture and interpret muscle function.
Keywords: biomechanics; functional morphology; locomotion; modelling; muscle; physiology.
© 2022 The Authors. Biological Reviews published by John Wiley & Sons Ltd on behalf of Cambridge Philosophical Society.
Figures









Similar articles
-
Computational modelling of muscle fibre operating ranges in the hindlimb of a small ground bird (Eudromia elegans), with implications for modelling locomotion in extinct species.PLoS Comput Biol. 2021 Apr 1;17(4):e1008843. doi: 10.1371/journal.pcbi.1008843. eCollection 2021 Apr. PLoS Comput Biol. 2021. PMID: 33793558 Free PMC article.
-
Direct intraoperative measurement of isometric contractile properties in living human muscle.J Physiol. 2023 May;601(10):1817-1830. doi: 10.1113/JP284092. Epub 2023 Apr 25. J Physiol. 2023. PMID: 36905200
-
Fibre operating lengths of human lower limb muscles during walking.Philos Trans R Soc Lond B Biol Sci. 2011 May 27;366(1570):1530-9. doi: 10.1098/rstb.2010.0345. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 21502124 Free PMC article.
-
How important are skeletal muscle mechanics in setting limits on jumping performance?J Exp Biol. 2007 Mar;210(Pt 6):923-33. doi: 10.1242/jeb.02731. J Exp Biol. 2007. PMID: 17337705 Review.
-
Techniques and applications of skeletal muscle diffusion tensor imaging: A review.J Magn Reson Imaging. 2016 Apr;43(4):773-88. doi: 10.1002/jmri.25016. Epub 2015 Jul 29. J Magn Reson Imaging. 2016. PMID: 26221741 Review.
Cited by
-
Gape drives regional variation in temporalis architectural dynamics in tufted capuchins.Philos Trans R Soc Lond B Biol Sci. 2023 Dec 4;378(1891):20220550. doi: 10.1098/rstb.2022.0550. Epub 2023 Oct 16. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37839440 Free PMC article.
-
Non invasive techniques for direct muscle quality assessment after exercise intervention in older adults: a systematic review.BMC Geriatr. 2024 Jul 31;24(1):642. doi: 10.1186/s12877-024-05243-3. BMC Geriatr. 2024. PMID: 39085773 Free PMC article.
-
Functional Tests of the Abdominal Wall Muscles in Normal Subjects and in Patients with Diastasis and Oblique Inguinal Hernias in a Pilot Study.J Funct Morphol Kinesiol. 2024 Sep 15;9(3):164. doi: 10.3390/jfmk9030164. J Funct Morphol Kinesiol. 2024. PMID: 39311272 Free PMC article.
-
Muscle-Driven Predictive Physics Simulations of Quadrupedal Locomotion in the Horse.Integr Comp Biol. 2024 Sep 27;64(3):694-714. doi: 10.1093/icb/icae095. Integr Comp Biol. 2024. PMID: 39003243 Free PMC article.
-
Static versus dynamic muscle modelling in extinct species: a biomechanical case study of the Australopithecus afarensis pelvis and lower extremity.PeerJ. 2024 Jan 31;12:e16821. doi: 10.7717/peerj.16821. eCollection 2024. PeerJ. 2024. PMID: 38313026 Free PMC article.
References
-
- * Abourachid, A. (1993). Mechanics of standing in birds: functional explanation of lameness problems in giant turkeys. British Poultry Science 34, 887–898. - PubMed
-
- * Abrams, G. D. , Ward, S. R. , Fridén, J. & Lieber, R. L. (2005). Pronator teres is an appropriate donor muscle for restoration of wrist and thumb extension. The Journal of Hand Surgery 30, 1068–1073. - PubMed
-
- * Abrams, R. A. , Tsai, A. M. , Watson, B. , Jamali, A. & Lieber, R. L. (2000). Skeletal muscle recovery after tenotomy and 7‐day delayed muscle length restoration. Muscle & Nerve 23, 707–714. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

