Cellular interplay between cardiomyocytes and non-myocytes in diabetic cardiomyopathy
- PMID: 35388880
- PMCID: PMC10153440
- DOI: 10.1093/cvr/cvac049
Cellular interplay between cardiomyocytes and non-myocytes in diabetic cardiomyopathy
Abstract
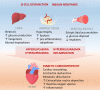
Patients with Type 2 diabetes mellitus (T2DM) frequently exhibit a distinctive cardiac phenotype known as diabetic cardiomyopathy. Cardiac complications associated with T2DM include cardiac inflammation, hypertrophy, fibrosis, and diastolic dysfunction in the early stages of the disease, which can progress to systolic dysfunction and heart failure. Effective therapeutic options for diabetic cardiomyopathy are limited and often have conflicting results. The lack of effective treatments for diabetic cardiomyopathy is due in part, to our poor understanding of the disease development and progression, as well as a lack of robust and valid preclinical human models that can accurately recapitulate the pathophysiology of the human heart. In addition to cardiomyocytes, the heart contains a heterogeneous population of non-myocytes including fibroblasts, vascular cells, autonomic neurons, and immune cells. These cardiac non-myocytes play important roles in cardiac homeostasis and disease, yet the effect of hyperglycaemia and hyperlipidaemia on these cell types is often overlooked in preclinical models of diabetic cardiomyopathy. The advent of human-induced pluripotent stem cells provides a new paradigm in which to model diabetic cardiomyopathy as they can be differentiated into all cell types in the human heart. This review will discuss the roles of cardiac non-myocytes and their dynamic intercellular interactions in the pathogenesis of diabetic cardiomyopathy. We will also discuss the use of sodium-glucose cotransporter 2 inhibitors as a therapy for diabetic cardiomyopathy and their known impacts on non-myocytes. These developments will no doubt facilitate the discovery of novel treatment targets for preventing the onset and progression of diabetic cardiomyopathy.
Keywords: Autonomic neurons; Cardiac fibroblasts; Cardiomyocytes; Diabetic cardiomyopathy; Endothelial cells; Immune cells.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures


References
-
- DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, Simonson DC, Testa MA, Weiss R. Type 2 diabetes mellitus. Nat Rev Dis Primers 2015;1:15019. - PubMed
-
- Htay T, Soe K, Lopez-Perez A, Doan AH, Romagosa MA, Aung K. Mortality and cardiovascular disease in type 1 and type 2 diabetes. Curr Cardiol Rep 2019;21:45. - PubMed
-
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

