A Mesoionic Diselenolene Anion and the Corresponding Radical Dianion
- PMID: 35388932
- PMCID: PMC9322424
- DOI: 10.1002/chem.202200893
A Mesoionic Diselenolene Anion and the Corresponding Radical Dianion
Abstract
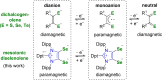
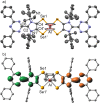
Dichalcogenolenes are archetypal redox non-innocent ligands with numerous applications. Herein, a diselenolene ligand with fundamentally different electronic properties is described. A mesoionic diselenolene was prepared by selenation of a C2-protected imidazolium salt. This ligand is diamagnetic, which is in contrast to the paramagnetic nature of standard dichalcogenolene monoanions. The new ligand is also redox-active, as demonstrated by isolation of a stable diselenolene radical dianion. The unique electronic properties of the new ligand give rise to unusual coordination chemistry. Thus, preparation of a hexacoordinate aluminum tris(diselenolene) complex and a Lewis acidic aluminate complex with two ligand-centered unpaired electrons was achieved.
Keywords: X-ray diffraction; aluminum complex; biradical; diselenolene; electronic structure; radical.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- None
-
- Kusamoto T., Nishihara H., Coord. Chem. Rev. 2019, 380, 419–439;
-
- Sproules S., Prog. Inorg. Chem. 2014, 58, 1–144;
-
- M. Arca, M. C. Aragoni, A. Pintus, 1, 2-Dichalcogenolene Ligands and Related Metal Complexes in Handbook of Chalcogen Chemistry, vol. 2 (Eds.: F. A. Devillanova, W-W. du Mont), RSC, 2013, 127–179;
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

