Emergence, Evolution, and Biological Characteristics of H10N4 and H10N8 Avian Influenza Viruses in Migratory Wild Birds Detected in Eastern China in 2020
- PMID: 35389243
- PMCID: PMC9045299
- DOI: 10.1128/spectrum.00807-22
Emergence, Evolution, and Biological Characteristics of H10N4 and H10N8 Avian Influenza Viruses in Migratory Wild Birds Detected in Eastern China in 2020
Abstract
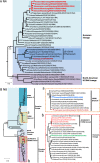
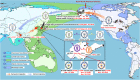
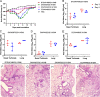
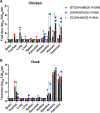
H10Nx influenza viruses have caused increasing public concern due to their occasional infection of humans. However, the genesis and biological characteristics of H10 viruses in migratory wild birds are largely unknown. In this study, we conducted active surveillance to monitor circulation of avian influenza viruses in eastern China and isolated five H10N4 and two H10N8 viruses from migratory birds in 2020. Genetic analysis indicated that the hemagglutinin (HA) genes of the seven H10 viruses were clustered into the North American lineage and established as a novel Eurasian branch in wild birds in South Korea, Bangladesh, and China. The neuraminidase (NA) genes of the H10N4 and H10N8 viruses originated from the circulating HxN4 and H5N8 viruses in migratory birds in Eurasia. We further revealed that some of the novel H10N4 and H10N8 viruses acquired the ability to bind human-like receptors. Animal studies indicated that these H10 viruses can replicate in mice, chickens, and ducks. Importantly, we found that the H10N4 and H10N8 viruses can transmit efficiently among chickens and ducks but induce lower HA inhibition (HI) antibody titers in ducks. These findings emphasized that annual surveillance in migratory waterfowl should be strengthened to monitor the introduction of wild-bird H10N4 and H10N8 reassortants into poultry. IMPORTANCE The emerging avian influenza reassortants and mutants in birds pose an increasing threat to poultry and public health. H10 avian influenza viruses are widely prevalent in wild birds, poultry, seals, and minks and pose an increasing threat to human health. The occasional human infections with H10N8 and H10N3 viruses in China have significantly increased public concern about the potential pandemic risk posed by H10 viruses. In this study, we found that the North American H10 viruses have been successfully introduced to Asia by migratory birds and further reassorted with other subtypes to generate novel H10N4 and H10N8 viruses in eastern China. These emerging H10 reassortants have a high potential to threaten the poultry industry and human health due to their efficient replication and transmission in chickens, ducks, and mice.
Keywords: H10N4; H10N8; avian influenza virus; replication and transmission; wild birds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Li X, Shi J, Guo J, Deng G, Zhang Q, Wang J, He X, Wang K, Chen J, Li Y, Fan J, Kong H, Gu C, Guan Y, Suzuki Y, Kawaoka Y, Liu L, Jiang Y, Tian G, Li Y, Bu Z, Chen H. 2014. Genetics, receptor binding property, and transmissibility in mammals of naturally isolated H9N2 Avian Influenza viruses. PLoS Pathog 10:e1004508. doi: 10.1371/journal.ppat.1004508. - DOI - PMC - PubMed
-
- Guo J, Wang Y, Zhao C, Gao X, Zhang Y, Li J, Wang M, Zhang H, Liu W, Wang C, Xia Y, Xu L, He G, Shen J, Sun X, Wang W, Han X, Zhang X, Hou Z, Jin X, Peng N, Li Y, Deng G, Cui P, Zhang Q, Li X, Chen H. 2021. Molecular characterization, receptor binding property, and replication in chickens and mice of H9N2 avian influenza viruses isolated from chickens, peafowls, and wild birds in eastern China. Emerg Microbes Infect 10:2098–2112. doi: 10.1080/22221751.2021.1999778. - DOI - PMC - PubMed
-
- Yin X, Deng G, Zeng X, Cui P, Hou Y, Liu Y, Fang J, Pan S, Wang D, Chen X, Zhang Y, Wang X, Tian G, Li Y, Chen Y, Liu L, Suzuki Y, Guan Y, Li C, Shi J, Chen H. 2021. Genetic and biological properties of H7N9 avian influenza viruses detected after application of the H7N9 poultry vaccine in China. PLoS Pathog 17:e1009561. doi: 10.1371/journal.ppat.1009561. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

