Microbial Biogeochemical Cycling of Nitrogen in Arid Ecosystems
- PMID: 35389249
- PMCID: PMC9199420
- DOI: 10.1128/mmbr.00109-21
Microbial Biogeochemical Cycling of Nitrogen in Arid Ecosystems
Abstract
Arid ecosystems cover ∼40% of the Earth's terrestrial surface and store a high proportion of the global nitrogen (N) pool. They are low-productivity, low-biomass, and polyextreme ecosystems, i.e., with (hyper)arid and (hyper)oligotrophic conditions and high surface UV irradiation and evapotranspiration. These polyextreme conditions severely limit the presence of macrofauna and -flora and, particularly, the growth and productivity of plant species. Therefore, it is generally recognized that much of the primary production (including N-input processes) and nutrient biogeochemical cycling (particularly N cycling) in these ecosystems are microbially mediated. Consequently, we present a comprehensive survey of the current state of knowledge of biotic and abiotic N-cycling processes of edaphic (i.e., open soil, biological soil crust, or plant-associated rhizosphere and rhizosheath) and hypo/endolithic refuge niches from drylands in general, including hot, cold, and polar desert ecosystems. We particularly focused on the microbially mediated biological nitrogen fixation, N mineralization, assimilatory and dissimilatory nitrate reduction, and nitrification N-input processes and the denitrification and anaerobic ammonium oxidation (anammox) N-loss processes. We note that the application of modern meta-omics and related methods has generated comprehensive data sets on the abundance, diversity, and ecology of the different N-cycling microbial guilds. However, it is worth mentioning that microbial N-cycling data from important deserts (e.g., Sahara) and quantitative rate data on N transformation processes from various desert niches are lacking or sparse. Filling this knowledge gap is particularly important, as climate change models often lack data on microbial activity and environmental microbial N-cycling communities can be key actors of climate change by producing or consuming nitrous oxide (N2O), a potent greenhouse gas.
Keywords: biogeochemistry; biological soil crusts; desert; diazotrophy; drylands; lithobiont; nitrogen cycling; soils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

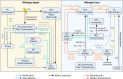

References
-
- IPCC. 2019. Summary for policymakers. In Shukla PR, Skea J, Calvo Buendia E, Masson-Delmotte V, Pörtner H-O, Roberts DC, Zhai P, Slade R, Connors S, van Diemen R, Ferrat M, Haughey E, Luz S, Neogi S, Pathak M, Petzold J, Portugal Pereira J, Vyas P, Huntley E, Kissick K, Belkacemi M, Malley J (ed), Climate change and land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems.
-
- Laity JJ. 2009. Deserts and desert environments, vol 3. Wiley-Blackwell, Hoboken, NJ.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

