Staphylococcus aureus Overcomes Anaerobe-Derived Short-Chain Fatty Acid Stress via FadX and the CodY Regulon
- PMID: 35389253
- PMCID: PMC9112931
- DOI: 10.1128/jb.00064-22
Staphylococcus aureus Overcomes Anaerobe-Derived Short-Chain Fatty Acid Stress via FadX and the CodY Regulon
Abstract
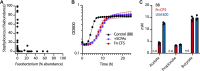
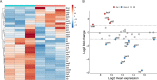
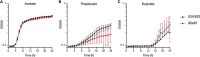
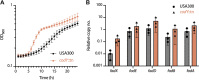
Chronic rhinosinusitis (CRS) is characterized by immune dysfunction, mucus hypersecretion, and persistent infection of the paranasal sinuses. While Staphylococcus aureus is a primary CRS pathogen, recent sequence-based surveys have found increased relative abundances of anaerobic bacteria, suggesting that S. aureus may experience altered metabolic landscapes in CRS relative to healthy airways. To test this possibility, we characterized the growth kinetics and transcriptome of S. aureus in supernatants of the abundant CRS anaerobe Fusobacterium nucleatum. While growth was initially delayed, S. aureus ultimately grew to similar levels as in the control medium. The transcriptome was significantly affected by F. nucleatum metabolites, with the agr quorum sensing system notably repressed. Conversely, expression of fadX, encoding a putative propionate coenzyme A (CoA)-transferase, was significantly increased, leading to our hypothesis that short-chain fatty acids (SCFAs) produced by F. nucleatum could mediate S. aureus growth behavior and gene expression. Supplementation with propionate and butyrate, but not acetate, recapitulated delayed growth phenotypes observed in F. nucleatum supernatants. A fadX mutant was found to be more sensitive than wild type to propionate, suggesting a role for FadX in the S. aureus SCFA stress response. Interestingly, spontaneous resistance to butyrate, but not propionate, was observed frequently. Whole-genome sequencing and targeted mutagenesis identified codY mutants as resistant to butyrate inhibition. Together, these data show that S. aureus physiology is dependent on its cocolonizing microbiota and metabolites they exchange and indicate that propionate and butyrate may act on different targets in S. aureus to suppress its growth. IMPORTANCE Staphylococcus aureus is an important CRS pathogen, and yet it is found in the upper airways of 30% to 50% of people without complications. The presence of strict and facultative anaerobic bacteria in CRS sinuses has recently spurred research into bacterial interactions and how they influence S. aureus physiology and pathogenesis. We show here that propionate and butyrate produced by one such CRS anaerobe, namely, Fusobacterium nucleatum, alter the growth and gene expression of S. aureus. We show that fadX is important for S. aureus to resist propionate stress and that the CodY regulon mediates growth in inhibitory concentrations of butyrate. This work highlights the possible complexity of S. aureus-anaerobe interactions and implicates membrane stress as a possible mechanism influencing S. aureus behavior in CRS sinuses.
Keywords: Fusobacterium; Staphylococcus aureus; anaerobes; microbiome; short-chain fatty acids; sinusitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
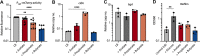
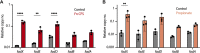

References
-
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, Bousquet PJ, Brozek G, Bruno A, Dahlén SE, Forsberg B, Gunnbjörnsdóttir M, Kasper L, Krämer U, Kowalski ML, Lange B, Lundbäck B, Salagean E, Todo-Bom A, Tomassen P, Toskala E, van Drunen CM, Bousquet J, Zuberbier T, Jarvis D, Burney P. 2011. Chronic rhinosinusitis in Europe—an underestimated disease. A GA2LEN study. Allergy 66:1216–1223. 10.1111/j.1398-9995.2011.02646.x. - DOI - PubMed
-
- Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, Batra PS, Bernal-Sprekelsen M, Bhattacharyya N, Chandra RK, Chiu A, Citardi MJ, Cohen NA, DelGaudio J, Desrosiers M, Dhong H-J, Douglas R, Ferguson B, Fokkens WJ, Georgalas C, Goldberg A, Gosepath J, Hamilos DL, Han JK, Harvey R, Hellings P, Hopkins C, Jankowski R, Javer AR, Kern R, Kountakis S, Kowalski ML, Lane A, Lanza DC, Lebowitz R, Lee H-M, Lin SY, Lund V, Luong A, Mann W, Marple BF, McMains KC, Metson R, Naclerio R, Nayak JV, Otori N, Palmer JN, Parikh SR, Passali D, Peters A, Piccirillo J, Poetker DM, Psaltis AJ, Ramadan HH, Ramakrishnan VR, Riechelmann H, et al.. 2016. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol 6:S22–209. 10.1002/alr.21695. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

