Cooperative Palladium/Isothiourea Catalyzed Enantioselective Formal (3+2) Cycloaddition of Vinylcyclopropanes and α,β-Unsaturated Esters
- PMID: 35389553
- PMCID: PMC9324207
- DOI: 10.1002/anie.202202621
Cooperative Palladium/Isothiourea Catalyzed Enantioselective Formal (3+2) Cycloaddition of Vinylcyclopropanes and α,β-Unsaturated Esters
Abstract
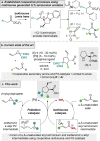
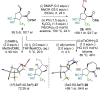
A protocol for the enantioselective synthesis of substituted vinylcyclopentanes has been realised using cooperative palladium and isothiourea catalysis. Treatment of vinylcyclopropanes with Pd(PPh3 )4 generates a zwitterionic π-allyl palladium intermediate that intercepts a catalytically generated α,β-unsaturated acyl ammonium species prepared from the corresponding α,β-unsaturated para-nitrophenyl ester and the isothiourea (R)-BTM. Intermolecular formal (3+2) cycloaddition between these reactive intermediates generates functionalised cyclopentanes in generally good yields and excellent diastereo- and enantiocontrol (up to >95 : 5 dr, 97 : 3 er), with the use of LiCl as an additive proving essential for optimal stereocontrol. To the best of our knowledge a dual transition metal/organocatalytic process involving α,β-unsaturated acyl ammonium intermediates has not been demonstrated previously.
Keywords: Cooperative Catalysis; Cycloaddition; Isothiourea; Palladium Catalysis; α,β-Unsaturated Acyl Ammonium.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

