Brain temperature affects quantitative features of hippocampal sharp wave ripples
- PMID: 35389772
- PMCID: PMC9109799
- DOI: 10.1152/jn.00047.2022
Brain temperature affects quantitative features of hippocampal sharp wave ripples
Abstract
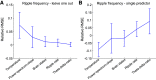
Biochemical mechanisms are temperature dependent. Brain temperature shows wide variations across brain states, and such changes may explain quantitative changes in network oscillations. Here, we report on the relationship between various hippocampal sharp wave ripple features to brain temperature. Ripple frequency, occurrence rate, and duration correlated with temperature dynamics. By focal manipulation of the brain temperature in the hippocampal CA1 region, we show that ripple frequency can be increased and decreased by local heating and cooling, respectively. Changes of other parameters, such as the rate of sharp wave-ripple complex (SPW-R) and ripple duration were not consistently affected. Our findings suggest that brain temperature in the CA1 region plays a leading role in affecting ripple frequency, whereas other parameters of SPW-Rs may be determined by mechanisms upstream from the CA1 region. These findings illustrate that physiological variations of brain temperature exert important effects on hippocampal circuit operations.NEW & NOTEWORTHY During physiological conditions, brain temperature fluctuates approximately 3°C between sleep and active waking. Here, we show that features of hippocampal ripples, including the rate of occurrence, peak frequency, and duration are correlated with brain temperature variations. Focal bidirectional manipulation of temperature in the hippocampal CA1 region in awake rodents show that ripple frequency can be altered in the direction expected from the correlational observations, implying that temperature plays a significant role.
Keywords: hippocampus; sharp wave ripples; temperature; thermal perturbation.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

