SARS-CoV-2-specific immune responses in boosted vaccine recipients with breakthrough infections during the Omicron variant surge
- PMID: 35389888
- PMCID: PMC9220829
- DOI: 10.1172/jci.insight.159474
SARS-CoV-2-specific immune responses in boosted vaccine recipients with breakthrough infections during the Omicron variant surge
Abstract
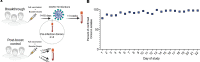
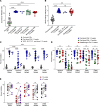
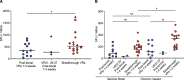
BackgroundBreakthrough SARS-CoV-2 infections in vaccinated individuals have been previously associated with suboptimal humoral immunity. However, less is known about breakthrough infections with the Omicron variant.MethodsWe analyzed SARS-CoV-2-specific antibody and cellular responses in healthy vaccine recipients who experienced breakthrough infections a median of 50 days after receiving a booster mRNA vaccine with an ACE2 binding inhibition assay and an ELISpot assay, respectively.ResultsWe found that high levels of antibodies inhibited vaccine strain spike protein binding to ACE2 but that lower levels inhibited Omicron variant spike protein binding to ACE2 in 4 boosted vaccine recipients prior to infection. The levels of antibodies that inhibited vaccine strain and Omicron spike protein binding after breakthrough in 18 boosted vaccine recipients were similar to levels seen in COVID-19-negative boosted vaccine recipients. In contrast, boosted vaccine recipients had significantly stronger T cell responses to both vaccine strain and Omicron variant spike proteins at the time of breakthrough.ConclusionOur data suggest that breakthrough infections with the Omicron variant can occur despite robust immune responses to the vaccine strain spike protein.FundingThis work was supported by the Johns Hopkins COVID-19 Vaccine-related Research Fund and by funds from the National Institute of Allergy and Infectious Disease intramural program as well as awards from the National Cancer Institute (U54CA260491) and the National Institutes of Allergy and Infectious Disease (K08AI156021 and U01AI138897).
Keywords: Adaptive immunity; COVID-19; T cells.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

