Emulsion-Based Postbiotic Formulation Is Comparable to Viable Cells in Eliciting a Localized Immune Response in Dairy Cows With Chronic Mastitis
- PMID: 35391729
- PMCID: PMC8981918
- DOI: 10.3389/fmicb.2022.759649
Emulsion-Based Postbiotic Formulation Is Comparable to Viable Cells in Eliciting a Localized Immune Response in Dairy Cows With Chronic Mastitis
Abstract
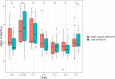
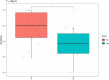
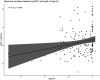
Bovine mastitis is a disease with a multi-etiological nature, defined as an infection and inflammation of the udder. Mastitis represents a significant ongoing concern in the dairy industry, leading to substantial losses in profits and revenue for farmers worldwide. The predominant causes of bovine mastitis include the pathogens Staphylococcus aureus, Streptococcus dysgalactiae, Streptococcus uberis, and Escherichia coli. Antibiotic administration is currently the main treatment option for mastitis. However, there is a pressing need for alternative therapies to treat and prevent the disease, especially with the emergence of antibiotic-resistant, mastitis-causing pathogens, resulting in antibiotic treatment failure. One such example is live bio-therapeutics (also known as probiotics), such as Lactococcus lactis DPC3147. The efficacy of this live bio-therapeutic has been demonstrated in several previous trials by our group. The most recent of these trials showed that an emulsion-based formulation of this strain was as effective as a commercial antibiotic formulation in treating sub-clinical and clinical cases of bovine mastitis. Here, we report the results of a follow-up field trial, in which we sought to gain insight into the mechanism of action of such live bio-therapeutics, focussing on chronic mastitis cases. We treated 28 cows with chronic mastitis with two separate emulsion-based formulations containing either viable L. lactis DPC3147 cells (15 cows) or heat-killed L. lactis DPC3147 cells (13 cows). We then evaluated the efficacies of the two formulations (two treatment groups) in terms of stimulating a localized immune response (quantified by measuring IL-8 concentrations in milk collected from udders affected by mastitis) and efficacies in terms of cure rates (quantified by reductions in somatic cell counts and absence of pathogens). We demonstrate that the presence of heat-inactivated bacteria (a postbiotic) was as effective as the live bio-therapeutic in eliciting a localized immune response in cows with chronic mastitis. The response to heat-killed cells (postbiotic) reported herein could have beneficial implications for farmers with regard to prolonging the shelf life of such emulsion-based formulations containing heat-killed cells of L. lactis DPC3147 for curing cows with mastitis.
Keywords: emulsion; lacticin 3147; live bio-therapeutic; mastitis; postbiotic; probiotics; somatic cell counts.
Copyright © 2022 Mathur, Linehan, Flynn, Byrne, Dillon, Conneely, Grimaud, Hill, Stanton and Ross.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
A Live Bio-Therapeutic for Mastitis, Containing Lactococcus lactis DPC3147 With Comparable Efficacy to Antibiotic Treatment.Front Microbiol. 2019 Sep 27;10:2220. doi: 10.3389/fmicb.2019.02220. eCollection 2019. Front Microbiol. 2019. PMID: 31611858 Free PMC article.
-
Microbial Aetiology, Antibiotic Susceptibility and Pathogen-Specific Risk Factors for Udder Pathogens from Clinical Mastitis in Dairy Cows.Animals (Basel). 2021 Jul 16;11(7):2113. doi: 10.3390/ani11072113. Animals (Basel). 2021. PMID: 34359241 Free PMC article.
-
Distribution of Lactococcus spp. in New York State dairy farms and the association of somatic cell count resolution and bacteriological cure in clinical mastitis samples.J Dairy Sci. 2020 Feb;103(2):1785-1794. doi: 10.3168/jds.2018-16199. Epub 2019 Dec 19. J Dairy Sci. 2020. PMID: 31864733
-
Lactococcus lactis, a bacterium with probiotic functions and pathogenicity.World J Microbiol Biotechnol. 2023 Sep 30;39(12):325. doi: 10.1007/s11274-023-03771-5. World J Microbiol Biotechnol. 2023. PMID: 37776350 Review.
-
Trends in diagnosis and control of bovine mastitis: a review.Pak J Biol Sci. 2013 Dec 1;16(23):1653-61. doi: 10.3923/pjbs.2013.1653.1661. Pak J Biol Sci. 2013. PMID: 24506032 Review.
Cited by
-
Postbiotics: an insightful review of the latest category in functional biotics.World J Microbiol Biotechnol. 2025 Aug 2;41(8):293. doi: 10.1007/s11274-025-04483-8. World J Microbiol Biotechnol. 2025. PMID: 40751848 Free PMC article. Review.
-
Potential Application of Postbiotics as a Natural Preservative in Cheese.Probiotics Antimicrob Proteins. 2025 May 6. doi: 10.1007/s12602-025-10559-6. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40327312 Review.
-
Development of innate immune memory by non-immune cells during Staphylococcus aureus infection depends on reactive oxygen species.Front Immunol. 2023 May 31;14:1138539. doi: 10.3389/fimmu.2023.1138539. eCollection 2023. Front Immunol. 2023. PMID: 37325649 Free PMC article.
-
Biomolecule-Producing Probiotic Bacterium Lactococcus lactis in Free or Nanoencapsulated Form for Endometritis Treatment and Fertility Improvement in Buffaloes.J Funct Biomater. 2024 May 21;15(6):138. doi: 10.3390/jfb15060138. J Funct Biomater. 2024. PMID: 38921512 Free PMC article.
References
LinkOut - more resources
Full Text Sources

