Activated CD8+CD38+ Cells Are Associated With Worse Clinical Outcome in Hospitalized COVID-19 Patients
- PMID: 35392095
- PMCID: PMC8982066
- DOI: 10.3389/fimmu.2022.861666
Activated CD8+CD38+ Cells Are Associated With Worse Clinical Outcome in Hospitalized COVID-19 Patients
Abstract
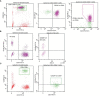
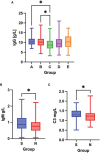
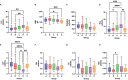
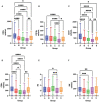
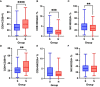
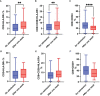
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), that spread around the world during the past 2 years, has infected more than 260 million people worldwide and has imposed an important burden on the healthcare system. Several risk factors associated with unfavorable outcome were identified, including elderly age, selected comorbidities, immune suppression as well as laboratory markers. The role of immune system in the pathophysiology of SARS-CoV-2 infection is indisputable: while an appropriate function of the immune system is important for a rapid clearance of the virus, progression to the severe and critical phases of the disease is related to an exaggerated immune response associated with a cytokine storm. We analyzed differences and longitudinal changes in selected immune parameters in 823 adult COVID-19 patients hospitalized in the Martin University Hospital, Martin, Slovakia. Examined parameters included the differential blood cell counts, various parameters of cellular and humoral immunity (serum concentration of immunoglobulins, C4 and C3), lymphocyte subsets (CD3+, CD4+, CD8+, CD19+, NK cells, CD4+CD45RO+), expression of activation (HLA-DR, CD38) and inhibition markers (CD159/NKG2A). Besides already known changes in the differential blood cell counts and basic lymphocyte subsets, we found significantly higher proportion of CD8+CD38+ cells and significantly lower proportion of CD8+NKG2A+ and NK NKG2A+ cells on admission in non-survivors, compared to survivors; recovery in survivors was associated with a significant increase in the expression of HLA-DR and with a significant decrease of the proportion of CD8+CD38+cells. Furthermore, patients with fatal outcome had significantly lower concentrations of C3 and IgM on admission. However, none of the examined parameters had sufficient sensitivity or specificity to be considered a biomarker of fatal outcome. Understanding the dynamic changes in immune profile of COVID-19 patients may help us to better understand the pathophysiology of the disease, potentially improve management of hospitalized patients and enable proper timing and selection of immunomodulator drugs.
Keywords: COVID-19; SARS-CoV-2; activated CD8+ cells; clinical outcome; immune cell dysregulation; immunologic predictors.
Copyright © 2022 Bobcakova, Barnova, Vysehradsky, Petriskova, Kocan, Diamant and Jesenak.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Worldometer . COVID-19 Coronavirus Pandemic (2022). Available at: https://www.worldometers.info/coronavirus/ (Accessed January 14, 2022).
-
- National Institute of Health . Clinical Spectrum of SARS-CoV-2 Infection (2021). Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (Accessed January 14 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

