Nintedanib and Dasatinib Treatments Induce Protective Autophagy as a Potential Resistance Mechanism in MPM Cells
- PMID: 35392170
- PMCID: PMC8982261
- DOI: 10.3389/fcell.2022.852812
Nintedanib and Dasatinib Treatments Induce Protective Autophagy as a Potential Resistance Mechanism in MPM Cells
Abstract
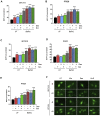
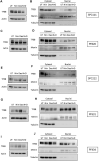
Malignant pleural mesothelioma (MPM) is a rare type of cancer with a grim prognosis. So far, no targetable oncogenic mutation was identified in MPM and biomarkers with predictive value toward drug sensitivity or resistance are also lacking. Nintedanib (BIBF1120) is a small-molecule tyrosine kinase inhibitor that showed promising efficacy preclinically and in phase II trial in MPM as an angiogenesis inhibitor combined with chemotherapy. However, the extended phase III trial failed. In this study, we investigated the effect of nintedanib on one of its targets, the SRC kinase, in two commercial and six novel MPM cell lines. Surprisingly, nintedanib treatment did not inhibit SRC activation in MPM cells and even increased phosphorylation of SRC in several cell lines. Combination treatment with the SRC inhibitor dasatinib could reverse this effect in all cell lines, however, the cellular response was dependent on the drug sensitivity of the cells. In 2 cell lines, with high sensitivity to both nintedanib and dasatinib, the drug combination had no synergistic effect but cell death was initiated. In 2 cell lines insensitive to nintedanib combination treatment reduced cell viability synergisticaly without cell death. In contrast, in these cells both treatments increased the autophagic flux assessed by degradation of the autophagy substrate p62 and increased presence of LC3B-II, increased number of GFP-LC3 puncta and decreased readings of the HiBiT-LC3 reporter. Additionaly, autophagy was synergistically promoted by the combined treatment. At the transcriptional level, analysis of lysosomal biogenesis regulator Transcription Factor EB (TFEB) showed that in all cell lines treated with nintedanib and to a lesser extent, with dasatinib, it became dephosphorylated and accumulated in the nucleus. Interestingly, the expression of certain known TFEB target genes implicated in autophagy or lysosomal biogenesis were significantly modified only in 1 cell line. Finally, we showed that autophagy induction in our MPM cell lines panel by nintedanib and dasatinib is independent of the AKT/mTOR and the ERK pathways. Our study reveals that autophagy can serve as a cytoprotective mechanism following nintedanib or dasatinib treatments in MPM cells.
Keywords: TFEB; autophagy; dasatinib; malignant pleural mesothelioma; nintedanib.
Copyright © 2022 Hegedüs, Szücs, Kudla, Heidenreich, Jendrossek, Peña-Llopis, Garay, Czirok, Aigner, Plönes, Vega-Rubin-de-Celis and Hegedüs.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Dasatinib modulates sensitivity to pemetrexed in malignant pleural mesothelioma cell lines.Oncotarget. 2016 Nov 22;7(47):76577-76589. doi: 10.18632/oncotarget.10428. Oncotarget. 2016. PMID: 27391433 Free PMC article.
-
Inhibition of c-Src expression and activation in malignant pleural mesothelioma tissues leads to apoptosis, cell cycle arrest, and decreased migration and invasion.Mol Cancer Ther. 2007 Jul;6(7):1962-72. doi: 10.1158/1535-7163.MCT-07-0052. Mol Cancer Ther. 2007. PMID: 17620427
-
MicroRNA-106a targets autophagy and enhances sensitivity of lung cancer cells to Src inhibitors.Lung Cancer. 2017 May;107:73-83. doi: 10.1016/j.lungcan.2016.06.004. Epub 2016 Jun 14. Lung Cancer. 2017. PMID: 27372519
-
[Systemic Treatment of Malignant Pleural Mesothelioma].Gan To Kagaku Ryoho. 2017 Dec;44(13):2041-2047. Gan To Kagaku Ryoho. 2017. PMID: 29361614 Review. Japanese.
-
Malignant peritoneal mesothelioma: a review.Transl Lung Cancer Res. 2018 Oct;7(5):537-542. doi: 10.21037/tlcr.2018.10.04. Transl Lung Cancer Res. 2018. PMID: 30450291 Free PMC article. Review.
Cited by
-
Is Autophagy Targeting a Valid Adjuvant Strategy in Conjunction with Tyrosine Kinase Inhibitors?Cancers (Basel). 2024 Aug 28;16(17):2989. doi: 10.3390/cancers16172989. Cancers (Basel). 2024. PMID: 39272847 Free PMC article. Review.
-
Polymeric Polylactic Acid-Glycolic Acid-Based Nanoparticles Deliver Nintedanib Across the Blood-Brain Barrier to Inhibit Glioblastoma Growth.Int J Mol Sci. 2025 Jan 7;26(2):443. doi: 10.3390/ijms26020443. Int J Mol Sci. 2025. PMID: 39859159 Free PMC article.
-
Lysosomes as a Target of Anticancer Therapy.Int J Mol Sci. 2023 Jan 22;24(3):2176. doi: 10.3390/ijms24032176. Int J Mol Sci. 2023. PMID: 36768500 Free PMC article. Review.
-
ATG5 as biomarker for early detection of malignant mesothelioma.BMC Res Notes. 2023 Apr 24;16(1):61. doi: 10.1186/s13104-023-06330-1. BMC Res Notes. 2023. PMID: 37095543 Free PMC article.
-
Post-Translational Modifications in Multiple Myeloma: Mechanisms of Drug Resistance and Therapeutic Opportunities.Biomolecules. 2025 May 12;15(5):702. doi: 10.3390/biom15050702. Biomolecules. 2025. PMID: 40427595 Free PMC article. Review.
References
-
- Amaravadi R. K., Kimmelman A. C., Debnath J. (2019). Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 9 (9), 1167–1181. 10.1158/2159-8290.CD-19-0292 - DOI - PMC - PubMed
-
- Dudek A. Z., Pang H., Kratzke R. A., Otterson G. A., Hodgson L., Vokes E. E., et al. (2012). Phase II Study of Dasatinib in Patients with Previously Treated Malignant Mesothelioma (Cancer and Leukemia Group B 30601): a Brief Report. J. Thorac. Oncol. 7 (4), 755–759. 10.1097/JTO.0b013e318248242c - DOI - PMC - PubMed
-
- Englinger B., Kallus S., Senkiv J., Heilos D., Gabler L., van Schoonhoven S., et al. (2017). Intrinsic Fluorescence of the Clinically Approved Multikinase Inhibitor Nintedanib Reveals Lysosomal Sequestration as Resistance Mechanism in FGFR-Driven Lung Cancer. J. Exp. Clin. Cancer Res. 36 (1), 122. 10.1186/s13046-017-0592-3 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

