Disrupting AMPK-Glycogen Binding in Mice Increases Carbohydrate Utilization and Reduces Exercise Capacity
- PMID: 35392375
- PMCID: PMC8980720
- DOI: 10.3389/fphys.2022.859246
Disrupting AMPK-Glycogen Binding in Mice Increases Carbohydrate Utilization and Reduces Exercise Capacity
Abstract
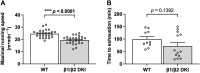
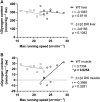
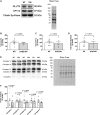
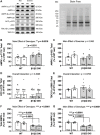
The AMP-activated protein kinase (AMPK) is a central regulator of cellular energy balance and metabolism and binds glycogen, the primary storage form of glucose in liver and skeletal muscle. The effects of disrupting whole-body AMPK-glycogen interactions on exercise capacity and substrate utilization during exercise in vivo remain unknown. We used male whole-body AMPK double knock-in (DKI) mice with chronic disruption of AMPK-glycogen binding to determine the effects of DKI mutation on exercise capacity, patterns of whole-body substrate utilization, and tissue metabolism during exercise. Maximal treadmill running speed and whole-body energy utilization during submaximal running were determined in wild type (WT) and DKI mice. Liver and skeletal muscle glycogen and skeletal muscle AMPK α and β2 subunit content and signaling were assessed in rested and maximally exercised WT and DKI mice. Despite a reduced maximal running speed and exercise time, DKI mice utilized similar absolute amounts of liver and skeletal muscle glycogen compared to WT. DKI skeletal muscle displayed reduced AMPK α and β2 content versus WT, but intact relative AMPK phosphorylation and downstream signaling at rest and following exercise. During submaximal running, DKI mice displayed an increased respiratory exchange ratio, indicative of greater reliance on carbohydrate-based fuels. In summary, whole-body disruption of AMPK-glycogen interactions reduces maximal running capacity and skeletal muscle AMPK α and β2 content and is associated with increased skeletal muscle glycogen utilization. These findings highlight potential unappreciated roles for AMPK in regulating tissue glycogen dynamics and expand AMPK's known roles in exercise and metabolism.
Keywords: AMP-activated protein kinase; carbohydrate binding module; energy utilization; exercise; glycogen; metabolism; skeletal muscle.
Copyright © 2022 Janzen, Whitfield, Murray-Segal, Kemp, Hawley and Hoffman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Metabolomics reveals mouse plasma metabolite responses to acute exercise and effects of disrupting AMPK-glycogen interactions.Front Mol Biosci. 2022 Aug 24;9:957549. doi: 10.3389/fmolb.2022.957549. eCollection 2022. Front Mol Biosci. 2022. PMID: 36090035 Free PMC article.
-
Mice with Whole-Body Disruption of AMPK-Glycogen Binding Have Increased Adiposity, Reduced Fat Oxidation and Altered Tissue Glycogen Dynamics.Int J Mol Sci. 2021 Sep 5;22(17):9616. doi: 10.3390/ijms22179616. Int J Mol Sci. 2021. PMID: 34502525 Free PMC article.
-
Genetic loss of AMPK-glycogen binding destabilises AMPK and disrupts metabolism.Mol Metab. 2020 Nov;41:101048. doi: 10.1016/j.molmet.2020.101048. Epub 2020 Jun 29. Mol Metab. 2020. PMID: 32610071 Free PMC article.
-
Interactive Roles for AMPK and Glycogen from Cellular Energy Sensing to Exercise Metabolism.Int J Mol Sci. 2018 Oct 26;19(11):3344. doi: 10.3390/ijms19113344. Int J Mol Sci. 2018. PMID: 30373152 Free PMC article. Review.
-
Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism.Proc Nutr Soc. 2011 Feb;70(1):92-9. doi: 10.1017/S0029665110003915. Epub 2010 Nov 11. Proc Nutr Soc. 2011. PMID: 21067629 Review.
Cited by
-
Exercise-Regulated Mitochondrial and Nuclear Signalling Networks in Skeletal Muscle.Sports Med. 2024 May;54(5):1097-1119. doi: 10.1007/s40279-024-02007-2. Epub 2024 Mar 25. Sports Med. 2024. PMID: 38528308 Free PMC article. Review.
-
Metabolomics reveals mouse plasma metabolite responses to acute exercise and effects of disrupting AMPK-glycogen interactions.Front Mol Biosci. 2022 Aug 24;9:957549. doi: 10.3389/fmolb.2022.957549. eCollection 2022. Front Mol Biosci. 2022. PMID: 36090035 Free PMC article.
-
The metabolic sensor AMPK: Twelve enzymes in one.Mol Metab. 2024 Dec;90:102042. doi: 10.1016/j.molmet.2024.102042. Epub 2024 Oct 2. Mol Metab. 2024. PMID: 39362600 Free PMC article. Review.
References
-
- Bergmeyer H. U. (1974). Methods of Enzymatic Analysis. Weinheim, New York, USA: Verlag Chemie; Academic Press.
-
- Camacho R. C., Galassetti P., Davis S. N., Wasserman D. H. (2005). Glucoregulation during and after Exercise in Health and Insulin-Dependent Diabetes. Exerc. Sport Sci. Rev. 33 (1), 17–23. - PubMed
LinkOut - more resources
Full Text Sources

