Vaccination Against SARS-CoV-2 Is Associated With a Lower Viral Load and Likelihood of Systemic Symptoms
- PMID: 35392460
- PMCID: PMC8982774
- DOI: 10.1093/ofid/ofac066
Vaccination Against SARS-CoV-2 Is Associated With a Lower Viral Load and Likelihood of Systemic Symptoms
Abstract
Background: Data conflict on whether vaccination decreases severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load. The objective of this analysis was to compare baseline viral load and symptoms between vaccinated and unvaccinated adults enrolled in a randomized trial of outpatient coronavirus disease 2019 (COVID-19) treatment.
Methods: Baseline data from the first 433 sequential participants enrolling into the COVID-OUT trial were analyzed. Adults aged 30-85 with a body mass index (BMI) ≥25 kg/m2 were eligible within 3 days of a positive SARS-CoV-2 test and <7 days of symptoms. Log10 polymerase chain reaction viral loads were normalized to human RNase P by vaccination status, by time from vaccination, and by symptoms.
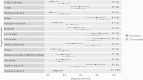
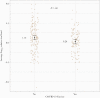
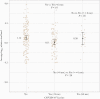
Results: Two hundred seventy-four participants with known vaccination status contributed optional nasal swabs for viral load measurement: median age, 46 years; median (interquartile range) BMI 31.2 (27.4-36.4) kg/m2. Overall, 159 (58%) were women, and 217 (80%) were White. The mean relative log10 viral load for those vaccinated <6 months from the date of enrollment was 0.11 (95% CI, -0.48 to 0.71), which was significantly lower than the unvaccinated group (P = .01). Those vaccinated ≥6 months before enrollment did not differ from the unvaccinated with respect to viral load (mean, 0.99; 95% CI, -0.41 to 2.40; P = .85). The vaccinated group had fewer moderate/severe symptoms of subjective fever, chills, myalgias, nausea, and diarrhea (all P < .05).
Conclusions: These data suggest that vaccination within 6 months of infection is associated with a lower viral load, and vaccination was associated with a lower likelihood of having systemic symptoms.
Keywords: SARS-CoV-2; symptoms; vaccines; viral load.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures

References
-
- Riemersma KK, Grogan BE, Kita-Yarbro A, et al. . Shedding of infectious SARS-CoV-2 despite vaccination. medRxiv 2021.2007.2031.21261387 [Preprint]. 24 August 2021. Available at: 10.1101/2021.07.31.21261387. Accessed 15 September 2021. - DOI
-
- Acharya CB, Schrom J, Mitchell AM, et al. . No significant difference in viral load between vaccinated and unvaccinated, asymptomatic and symptomatic groups when infected with SARS-CoV-2 Delta variant. medRxiv 2021.2009.2028.21264262 [Preprint]. 29 September 2021. Available at: 10.1101/2021.09.28.21264262. Accessed 20 October 2021. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

