Investigating the Implications of CFTR Exon Skipping Using a Cftr Exon 9 Deleted Mouse Model
- PMID: 35392567
- PMCID: PMC8981082
- DOI: 10.3389/fphar.2022.868863
Investigating the Implications of CFTR Exon Skipping Using a Cftr Exon 9 Deleted Mouse Model
Abstract
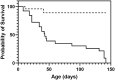
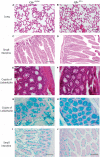
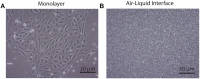
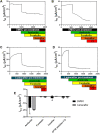
Introduction: Severity and disease progression in people with Cystic Fibrosis (CF) is typically dependent on their genotype. One potential therapeutic strategy for people with specific mutations is exon skipping with antisense oligonucleotides (AO). CFTR exon 9 is an in-frame exon and hence the exclusion of this exon would excise only 31 amino acids but not alter the reading frame of the remaining mRNA. Splice mutations 1209 + 1 G > C and 1209 + 2 T > G were documented to cause CFTR exon 9 skipping and these variants were reported to manifest as a milder CF disease, therefore exon 9 skipping could be beneficial for people with class I mutations that affect exon 9 such as p.Trp401X. While the impact of exon 9 skipping on gene expression and cellular pathways can be studied in cells in vitro, trace amount of full-length normal or mutated material could confound the evaluation. To overcome this limitation, the impact of CFTR exon 9 skipping on disease phenotype and severity is more effectively evaluated in a small animal model. It was hypothesised that antisense oligonucleotide-mediated skipping this particular exon could result in a "mild mouse CF phenotype". Methods: Cftr exon 9 deleted mice were generated using homologous recombination. Survival of homozygous (Cftr Δ9/Δ9 ) and heterozygous (Cftr Δ9/+ ) mice was compared to that of other CF mouse models, and lung and intestinal organ histology examined for any pathologies. Primary airway epithelial cells (pAECs) were harvested from Cftr Δ9/Δ9 mice and cultured at the Air Liquid Interface for CFTR functional assessment using Ussing Chamber analysis. Results: A Cftr Δ9/Δ9 mouse model presented with intestinal obstructions, and at time of weaning (21 days). Cftr Δ9/Δ9 mice had a survival rate of 83% that dropped to 38% by day 50. Histological sections of the small intestine from Cftr Δ9/Δ9 mice showed more goblet cells and mucus accumulation than samples from the Cftr Δ9/+ littermates. Airway epithelial cell cultures established from Cftr Δ9/Δ9 mice were not responsive to forskolin stimulation. Summary: The effect of Cftr exon 9 deletion on Cftr function was assessed and it was determined that the encoded Cftr isoform did not result in a milder "mouse CF disease phenotype," suggesting that Cftr exon 9 is not dispensable, although further investigation in human CF pAECs would be required to confirm this observation.
Keywords: cystic fibrosis transmembrane conductance regulator; exon deletion; exon skipping therapy; mouse model; transgenic mouse.
Copyright © 2022 Martinovich, Kicic, Stick, Johnsen, Fletcher and Wilton.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Clarke L. L., Grubb B. R., Yankaskas J. R., Cotton C. U., McKenzie A., Boucher R. C. (1994). Relationship of a Non-cystic Fibrosis Transmembrane Conductance Regulator-Mediated Chloride Conductance to Organ-Level Disease in Cftr(-/-) Mice. Proc. Natl. Acad. Sci. U S A. 91 (2), 479–483. 10.1073/pnas.91.2.479 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

