Glucose-to-Resistor Transduction Integrated into a Radio-Frequency Antenna for Chip-less and Battery-less Wireless Sensing
- PMID: 35392657
- PMCID: PMC9040053
- DOI: 10.1021/acssensors.2c00394
Glucose-to-Resistor Transduction Integrated into a Radio-Frequency Antenna for Chip-less and Battery-less Wireless Sensing
Abstract
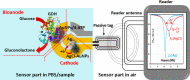
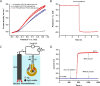
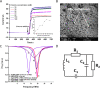
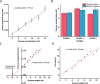
To maximize the potential of 5G infrastructure in healthcare, simple integration of biosensors with wireless tag antennas would be beneficial. This work introduces novel glucose-to-resistor transduction, which enables simple, wireless biosensor design. The biosensor was realized on a near-field communication tag antenna, where a sensing bioanode generated electrical current and electroreduced a nonconducting antenna material into an excellent conductor. For this, a part of the antenna was replaced by a Ag nanoparticle layer oxidized to high-resistance AgCl. The bioanode was based on Au nanoparticle-wired glucose dehydrogenase (GDH). The exposure of the cathode-bioanode to glucose solution resulted in GDH-catalyzed oxidation of glucose at the bioanode with a concomitant reduction of AgCl to highly conducting Ag on the cathode. The AgCl-to-Ag conversion strongly affected the impedance of the antenna circuit, allowing wireless detection of glucose. Mimicking the final application, the proposed wireless biosensor was ultimately evaluated through the measurement of glucose in whole blood, showing good agreement with the values obtained with a commercially available glucometer. This work, for the first time, demonstrates that making a part of the antenna from the AgCl layer allows achieving simple, chip-less, and battery-less wireless sensing of enzyme-catalyzed reduction reaction.
Keywords: Internet of Things; chip-less wireless sensing; direct electron transfer; glucose dehydrogenase; wireless detection of glucose.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Arefin M. S.; Redoute J. M.; Yuce M. R. Wireless biosensors for POC medical applications. Woodhead Publ. Ser. Biomater. 2017, 118, 151–180.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

