Comparison of Clinical and Imaging Outcomes of Different Doses of Adipose-Derived Stromal Vascular Fraction Cell Treatment for Knee Osteoarthritis
- PMID: 35392685
- PMCID: PMC9003644
- DOI: 10.1177/09636897211067454
Comparison of Clinical and Imaging Outcomes of Different Doses of Adipose-Derived Stromal Vascular Fraction Cell Treatment for Knee Osteoarthritis
Abstract
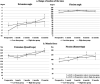
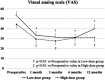
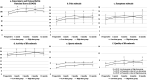
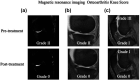
Favorable clinical outcomes of intra-articular injection of adipose-derived stromal vascular fraction (SVF) cells for knee osteoarthritis (OA) have been reported, but the effects of different doses of SVF cells have not been examined. This study aimed to compare the short-term clinical and imaging outcomes of different doses of SVF cells for knee OA treatment. This study included 60 patients with knee OA who underwent intra-articular injection of SVF cells. The follow-up period was at least 12 months. Thirty patients received an intra-articular injection of 2.5×107 SVF cells (low-dose group), and the remaining 30 patients received an intra-articular injection of 5.0×107 SVF cells (high-dose group). Clinical evaluations were performed for the Knee injury and Osteoarthritis Outcome Score (KOOS). Imaging evaluations, including the magnetic resonance imaging Osteoarthritis Knee Score (MOAKS) features (bone marrow lesions, cartilage defects, osteophytes, Hoffa's synovitis, and effusion synovitis), were also performed. All clinical and imaging evaluations were performed preoperatively and 12 months postoperatively and compared between the groups. In demographic data, no significant differences were found between the two groups. The total score of KOOS at 12 months postoperatively was significantly more favorable than the preoperative score in the high-dose groups. Pain and symptoms subscale scores of KOOS at 12 months postoperatively were significantly better in the high-dose group than in the low-dose group. The bone marrow lesions, Hoffa's synovitis, and effusion synovitis improved approximately 30-40% at 12 months postoperatively compared to baseline in both groups. However, there were no significant differences in imaging evaluations between the two groups. In conclusion, the pain and symptoms subscale scores of KOOS from baseline to 12 months postoperatively improved better in the high-dose group than in the low-dose group. Our findings suggest that intra-articular injection of SVF cells for knee OA is an innovative approach.
Keywords: adipose-derived stromal vascular fraction cells; dose-effect; magnetic resonance imaging osteoarthritis knee score; osteoarthritis; stem cell.
Figures


Similar articles
-
The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis.BMC Musculoskelet Disord. 2020 Apr 6;21(1):207. doi: 10.1186/s12891-020-03231-3. BMC Musculoskelet Disord. 2020. PMID: 32252731 Free PMC article.
-
Comparative Clinical Outcomes After Intra-articular Injection With Adipose-Derived Cultured Stem Cells or Noncultured Stromal Vascular Fraction for the Treatment of Knee Osteoarthritis.Am J Sports Med. 2019 Sep;47(11):2577-2583. doi: 10.1177/0363546519864359. Epub 2019 Aug 2. Am J Sports Med. 2019. PMID: 31373830
-
Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial.Int Orthop. 2019 May;43(5):1123-1134. doi: 10.1007/s00264-018-4099-0. Epub 2018 Aug 14. Int Orthop. 2019. PMID: 30109404 Clinical Trial.
-
Clinical Efficacy of Bone Marrow Aspirate Concentrate Versus Stromal Vascular Fraction Injection in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis.Am J Sports Med. 2022 Apr;50(5):1451-1461. doi: 10.1177/03635465211014500. Epub 2021 Jun 8. Am J Sports Med. 2022. PMID: 34102078
-
Intra-articular Injection of Autologous Adipose-Derived Stem Cells or Stromal Vascular Fractions: Are They Effective for Patients With Knee Osteoarthritis? A Systematic Review With Meta-analysis of Randomized Controlled Trials.Am J Sports Med. 2023 Mar;51(3):837-848. doi: 10.1177/03635465211053893. Epub 2022 Jan 12. Am J Sports Med. 2023. PMID: 35019764
Cited by
-
Safety and efficacy of autologous adipose-derived stem cells for knee osteoarthritis in the elderly population: A systematic review.J Clin Orthop Trauma. 2024 Nov 7;59:102804. doi: 10.1016/j.jcot.2024.102804. eCollection 2024 Dec. J Clin Orthop Trauma. 2024. PMID: 39628863 Review.
-
Adipose Tissue-Derived Therapies for Osteoarthritis: Multifaceted Mechanisms and Clinical Prospects.Cells. 2025 May 2;14(9):669. doi: 10.3390/cells14090669. Cells. 2025. PMID: 40358193 Free PMC article. Review.
-
Stem cells for the treatment of early to moderate osteoarthritis of the knee: a systematic review.J Exp Orthop. 2023 Oct 7;10(1):102. doi: 10.1186/s40634-023-00665-1. J Exp Orthop. 2023. PMID: 37804354 Free PMC article. Review.
-
Injection of Autologous Adipose Stromal Vascular Fraction in Combination with Autologous Conditioned Plasma for the Treatment of Advanced Knee Osteoarthritis Significantly Improves Clinical Symptoms.J Clin Med. 2024 May 22;13(11):3031. doi: 10.3390/jcm13113031. J Clin Med. 2024. PMID: 38892743 Free PMC article.
-
A Worldwide Analysis of Adipose-Derived Stem Cells and Stromal Vascular Fraction in Orthopedics: Current Evidence and Applications.J Clin Med. 2023 Jul 17;12(14):4719. doi: 10.3390/jcm12144719. J Clin Med. 2023. PMID: 37510834 Free PMC article. Review.
References
-
- Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis - an untreatable disease? Nat Rev Drug Discov. 2005;4(4):331–344. - PubMed
-
- Leardini G, Salaffi F, Caporali R, Canesi B, Rovati L, Montanelli R. Direct and indirect costs of osteoarthritis of the knee. Clin Exp Rheumatol. 2004;22(6):699–706. - PubMed
-
- Mündermann A, Nigg BM, Humble RN, Stefanyshyn DJ. Orthotic comfort is related to kinematics, kinetics, and EMG in recreational runners. Med Sci Sports Exerc. 2003;35(10):1710–1719. - PubMed
-
- Messier SP, Loeser RF, Hoover JL, Semble EL, Wise CM. Osteoarthritis of the knee: effects on gait, strength, and flexibility. Arch Phys Med Rehabil. 1992;73(1):29–36. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

