IgA nephropathy relapse following COVID-19 vaccination treated with corticosteroid therapy: case report
- PMID: 35392838
- PMCID: PMC8988530
- DOI: 10.1186/s12882-022-02769-9
IgA nephropathy relapse following COVID-19 vaccination treated with corticosteroid therapy: case report
Abstract
Background: The flare of immune-mediated disease following coronavirus disease of 2019 (COVID-19) vaccination is a rare adverse event following immunization. De novo, as well as relapsing IgA nephropathy (IgAN) cases, have been reported following either mRNA-1273 (Moderna) or BNT162b2 (Pfizer-BioNTech) vaccination. To our knowledge, the majority of IgAN relapses did not result in severe acute kidney injury (AKI) and resolved spontaneously.
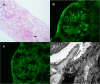
Case presentation: This is a case of a 54-year-old female with a previous diagnosis of IgAN who developed IgAN relapse following the second dose of Moderna vaccine. Gross hematuria developed 2 days after vaccination, which was accompanied by significant AKI. Kidney biopsy showed mild tubular atrophy and IgA staining in mesangium without crescent formation. Significant improvement in serum creatinine (Cr) was observed on day 10 after initiating prednisone. Cr came back to normal within 3 months after initiating corticosteroid.
Conclusion: COVID-19 vaccination is associated with a flare of IgAN that may cause significant AKI. Steroid therapy is associated with recovery. IgAN flare after COVID-19 vaccination should be closely monitored to elucidate any adverse effect associated with the novel vaccine.
Keywords: AKI; COVID-19 vaccine; Hematuria; IgA nephropathy; Kidney biopsy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

