Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons
- PMID: 35392976
- PMCID: PMC8991803
- DOI: 10.1186/s12964-022-00854-y
Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons
Abstract
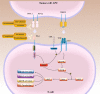
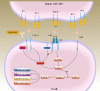
The main breakthrough in tumor immunotherapy was the discovery of immune checkpoint (IC) proteins, which act as a potent suppressor of the immune system by a myriad of mechanisms. After that, scientists focused on the immune checkpoint molecules mainly. Thereby, much effort was spent to progress novel strategies for suppressing these inhibitory axes, resulting in the evolution of immune checkpoint inhibitors (ICIs). Then, ICIs have become a promising approach and shaped a paradigm shift in tumor immunotherapies. CTLA-4 plays an influential role in attenuation of the induction of naïve and memory T cells by engagement with its responding ligands like B7-1 (CD80) and B7-2 (CD86). Besides, PD-1 is predominantly implicated in adjusting T cell function in peripheral tissues through its interaction with programmed death-ligand 1 (PD-L1) and PD-L2. Given their suppressive effects on anti-tumor immunity, it has firmly been documented that ICIs based therapies can be practical and rational therapeutic approaches to treat cancer patients. Nonetheless, tumor inherent or acquired resistance to ICI and some treatment-related toxicities restrict their application in the clinic. The current review will deliver a comprehensive overview of the ICI application to treat human tumors alone or in combination with other modalities to support more desired outcomes and lower toxicities in cancer patients. Video Abstract.
Keywords: CTLA-4; Cancer; Immune checkpoint inhibitors; Immunotherapy; PD-1/PD-L1.
© 2022. The Author(s).
Conflict of interest statement
All authors certify that no competing interests exists in this manuscript.
Figures
References
-
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. - PubMed
-
- Naidoo J, Page DB, Wolchok JD. Immune checkpoint blockade. Hematol Oncol Clin. 2014;28(3):585–600. - PubMed
-
- Toor SM, Nair VS, Decock J, Elkord E, editors. Immune checkpoints in the tumor microenvironment. Seminars in cancer biology. Amsterdam: Elsevier; 2020. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

