Obesity I: Overview and molecular and biochemical mechanisms
- PMID: 35393120
- PMCID: PMC9050949
- DOI: 10.1016/j.bcp.2022.115012
Obesity I: Overview and molecular and biochemical mechanisms
Abstract
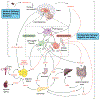
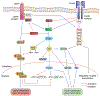
Obesity is a chronic, relapsing condition characterized by excess body fat. Its prevalence has increased globally since the 1970s, and the number of obese and overweight people is now greater than those underweight. Obesity is a multifactorial condition, and as such, many components contribute to its development and pathogenesis. This is the first of three companion reviews that consider obesity. This review focuses on the genetics, viruses, insulin resistance, inflammation, gut microbiome, and circadian rhythms that promote obesity, along with hormones, growth factors, and organs and tissues that control its development. It shows that the regulation of energy balance (intake vs. expenditure) relies on the interplay of a variety of hormones from adipose tissue, gastrointestinal tract, pancreas, liver, and brain. It details how integrating central neurotransmitters and peripheral metabolic signals (e.g., leptin, insulin, ghrelin, peptide YY3-36) is essential for controlling energy homeostasis and feeding behavior. It describes the distinct types of adipocytes and how fat cell development is controlled by hormones and growth factors acting via a variety of receptors, including peroxisome proliferator-activated receptor-gamma, retinoid X, insulin, estrogen, androgen, glucocorticoid, thyroid hormone, liver X, constitutive androstane, pregnane X, farnesoid, and aryl hydrocarbon receptors. Finally, it demonstrates that obesity likely has origins in utero. Understanding these biochemical drivers of adiposity and metabolic dysfunction throughout the life cycle lends plausibility and credence to the "obesogen hypothesis" (i.e., the importance of environmental chemicals that disrupt these receptors to promote adiposity or alter metabolism), elucidated more fully in the two companion reviews.
Keywords: Adipose tissue; Energy balance; Hormone receptors; Metabolism; Microbiome; Obesity.
Crown Copyright © 2022. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Jastreboff AM, Kotz CM, Kahan S, Kelly AS, Heymsfield SB, Obesity as a Disease: The Obesity Society 2018 Position Statement, Obesity (Silver Spring) 27(1) (2019) 7–9. - PubMed
-
- Mechanick JI, Garber AJ, Handelsman Y, Garvey WT, American Association of Clinical Endocrinologists’ position statement on obesity and obesity medicine, Endocr Pract 18(5) (2012) 642–8. - PubMed
-
- Blüher M, Obesity: global epidemiology and pathogenesis, Nat Rev Endocrinol 15(5) (2019) 288–298. - PubMed
-
- Morales Camacho WJ, Molina Díaz JM, Plata Ortiz S, Plata Ortiz JE, Morales Camacho MA, Calderón BP, Childhood obesity: Aetiology, comorbidities, and treatment, Diabetes Metab Res Rev 35(8) (2019) e3203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES025128/ES/NIEHS NIH HHS/United States
- P30 ES030283/ES/NIEHS NIH HHS/United States
- P30 DK063720/DK/NIDDK NIH HHS/United States
- P30 ES005022/ES/NIEHS NIH HHS/United States
- P01 ES028942/ES/NIEHS NIH HHS/United States
- T32 ES011564/ES/NIEHS NIH HHS/United States
- R21 ES031510/ES/NIEHS NIH HHS/United States
- R01 ES032189/ES/NIEHS NIH HHS/United States
- P20 GM103641/GM/NIGMS NIH HHS/United States
- P01 AT003961/AT/NCCIH NIH HHS/United States
- R01 MH123544/MH/NIMH NIH HHS/United States
- R00 ES030405/ES/NIEHS NIH HHS/United States
- R35 ES028373/ES/NIEHS NIH HHS/United States
- P42 ES023716/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

