Potency, toxicity and protection evaluation of PastoCoAd candidate vaccines: Novel preclinical mix and match rAd5 S, rAd5 RBD-N and SOBERANA dimeric-RBD protein
- PMID: 35393148
- PMCID: PMC8977851
- DOI: 10.1016/j.vaccine.2022.03.066
Potency, toxicity and protection evaluation of PastoCoAd candidate vaccines: Novel preclinical mix and match rAd5 S, rAd5 RBD-N and SOBERANA dimeric-RBD protein
Erratum in
-
Corrigendum to "Potency, toxicity and protection evaluation of PastoCoAd candidate vaccines: Novel preclinical mix and match rAd5 S, rAd5 RBD-N and SOBERANA dimeric-RBD protein" [Vaccine 40(20) (2022) 2856-2868].Vaccine. 2022 Nov 15;40(48):7009. doi: 10.1016/j.vaccine.2022.10.019. Epub 2022 Oct 28. Vaccine. 2022. PMID: 36374711 Free PMC article. No abstract available.
Abstract
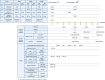
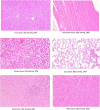
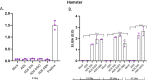
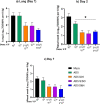
Despite substantial efforts, no effective treatment has been discovered for severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) infection. Therefore, vaccination to reach herd immunity is the ultimate solution to control the coronavirus disease 2019 (COVID-19) pandemic. This study aimed to evaluate the potency, toxicity, and protection of candidate PastoCoAd vaccines as novel mix and match of recombinant adenovirus type 5 (rAd5) containing the full-length spike protein (rAd5-S), rAd5 containing the receptor-binding domain of S protein and nucleoprotein (rAd5 RBD-N), and SOBERANA dimeric RBD protein of SARS-CoV-2. Three vaccine candidates were developed against SARS-CoV-2 using adenoviral vectors, including the prime-boost (rAd5-S/rAd5 RBD-N), heterologous prime-boost (rAd5-S/ SOBERANA vaccine), and prime only (mixture of rAd5-S and rAd5 RBD-N). The rAd5-S and rAd5 RBD-N were produced with a Cytomegalovirus promoter and the human tissue plasminogen activator (tPA) leader sequence. The immunogenicity of vaccine candidates was also evaluated in mouse, rabbit, and hamster models and protection was evaluated in a hamster model. Following the injection of vaccine candidates, no significant toxicity was observed in the tissues of animal models. The immunogenicity studies of mice, rabbits, and hamsters showed that responses of total IgG antibodies were significantly higher with the prime-only and heterologous prime-boost vaccines as compared to the other groups (P < 0.009). Virus neutralizing antibodies were detected, and the level of cytokines related to humoral and cellular immunity increased significantly in all vaccinated models. A high cellular immunity response was found in the vaccinated groups compared to the controls. On the other hand, the vaccine challenge test showed that the virus titers significantly decreased in the pharynx and lung tissues of vaccinated hamsters compared to the control group. These successful findings suggest the safety and protection produced by the heterologous prime-boost vaccine (adenovector/ SOBERANA RBD), as well as a single dose of adenovector vaccine in animal models.
Keywords: Adenovector; COVID-19; Iran; Protection; Safety; Vaccine.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Stability of Neutralizing Antibody of PastoCoAd Vaccine Candidates against a Variant of Concern of SARS-CoV-2 in Animal Models.Iran Biomed J. 2024 Jul 1;28(4):214-20. doi: 10.61186/ibj.3980. Iran Biomed J. 2024. PMID: 39044638 Free PMC article.
-
Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia.Lancet. 2020 Sep 26;396(10255):887-897. doi: 10.1016/S0140-6736(20)31866-3. Epub 2020 Sep 4. Lancet. 2020. PMID: 32896291 Free PMC article. Clinical Trial.
-
Safety and Efficacy of Combined Intramuscular/Intranasal RAZI-COV PARS Vaccine Candidate Against SARS-CoV-2: A Preclinical Study in Several Animal Models.Front Immunol. 2022 May 26;13:836745. doi: 10.3389/fimmu.2022.836745. eCollection 2022. Front Immunol. 2022. PMID: 35693788 Free PMC article.
-
Studies of SARS virus vaccines.Hong Kong Med J. 2008 Aug;14 Suppl 4:39-43. Hong Kong Med J. 2008. PMID: 18708674 Review.
-
'Mix and Match' vaccination: Is dengue next?Vaccine. 2022 Oct 26;40(45):6455-6462. doi: 10.1016/j.vaccine.2022.09.007. Epub 2022 Oct 1. Vaccine. 2022. PMID: 36195473 Free PMC article. Review.
Cited by
-
Comparative assessment of a COVID-19 vaccine after technology transfer to Iran from critical quality attributes to clinical and immunogenicity aspects.Sci Rep. 2024 Nov 5;14(1):26793. doi: 10.1038/s41598-024-77331-8. Sci Rep. 2024. PMID: 39501012 Free PMC article.
-
Stability of Neutralizing Antibody of PastoCoAd Vaccine Candidates against a Variant of Concern of SARS-CoV-2 in Animal Models.Iran Biomed J. 2024 Jul 1;28(4):214-20. doi: 10.61186/ibj.3980. Iran Biomed J. 2024. PMID: 39044638 Free PMC article.
-
Experimental Validation of MHC Class I and II Peptide-Based Potential Vaccine Candidates for Human Papilloma Virus Using Sprague-Dawly Models.Molecules. 2023 Feb 10;28(4):1687. doi: 10.3390/molecules28041687. Molecules. 2023. PMID: 36838675 Free PMC article.
-
Lessons from COVID-19 Pandemic: A Successful Policy and Practice by Pasteur Institute of Iran.Iran Biomed J. 2024 Jan 1;28(1):1-7. doi: 10.61186/ibj.3964. Epub 2023 Oct 11. Iran Biomed J. 2024. PMID: 38224028 Free PMC article.
-
A review of the scientific literature on experimental toxicity studies of COVID-19 vaccines, with special attention to publications in toxicology journals.Arch Toxicol. 2024 Nov;98(11):3603-3617. doi: 10.1007/s00204-024-03854-8. Epub 2024 Sep 3. Arch Toxicol. 2024. PMID: 39225797 Free PMC article. Review.
References
-
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

