Multisite prospective Liver Disease and Reproductive Ageing (LIVRA) study in US women living with and without HIV
- PMID: 35393310
- PMCID: PMC8991036
- DOI: 10.1136/bmjopen-2021-055706
Multisite prospective Liver Disease and Reproductive Ageing (LIVRA) study in US women living with and without HIV
Abstract
Purpose: The Liver Disease and Reproductive Ageing (LIVRA) study leverages the infrastructure of the decades-long multicentre prospective Women's Interagency HIV Study (WIHS) to examine the contributions of HIV, hepatitis C virus (HCV) and ageing to liver disease progression in women.
Participants: From 2013 to 2018, LIVRA enrolled 1576 participants (77 HCV-seropositive only, 248 HIV/HCV-seropositive, 868 HIV-seropositive only and 383 HIV/HCV-seronegative) who underwent vibration controlled transient elastography (VCTE). A VCTE quality assurance programme was established to ensure consistency and accuracy for longitudinal assessment of steatosis (fatty liver) via the controlled attenuation parameter (CAP) and fibrosis via liver stiffness (LS). Demographic, lifestyle factors, anthropometry, clinical and medication history, host genetics, immune markers and hormone levels were collected as part of the WIHS.
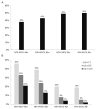
Findings to date: At baseline, 737 of 1543 women with CAP measurements had steatosis (CAP ≥248 dB/m) and 375 of 1576 women with LS measurements had significant fibrosis (LS ≥7.1 kPa), yielding a prevalence of 48% and 24%, respectively. On multivariable analysis, waist circumference (WC) and insulin resistance were independently associated with higher CAP (17.8 dB/m per 10 cm (95% CI:16.2 to 19.5) and 1.2 dB/m per doubling (95% CI:0.8 to 1.6), respectively). By contrast, HIV/HCV seropositivity and HCV seropositivity alone were associated with less steatosis compared with HIV/HCV-seronegative women, although the latter did not reach statistical significance (-9.2 dB/m (95% CI:-18.2 to -0.3) and -10.4 dB/m (95% CI: -23.8 to 3.1), respectively). Factors independently associated with higher LS were age (4.4% per 10 years (95% CI: 0.4% to 8.4%)), WC (5.0% per 10 cm (95% CI: 3.3% to 6.6%)), CAP steatosis (0.6% per 10 dB/m (95% CI: 0.1% to 1.0%)), HIV/HCV seropositivity (33% (95% CI: 24% to 44%)) and HCV seropositivity alone (43% (95% CI: 28% to 60%)). Excluding scans that were invalid based on traditional criteria for unreliability did not affect the results.
Future plans: Enrolled women undergo VCTE at 3-year intervals unless LS is ≥9.5 kPa, indicating advanced fibrosis, in which case VCTE is performed annually. Participants also undergo VCTE every 6 months until 18 months after HCV treatment initiation. Analysis of the data collected will provide insights into the impact of ageing/ovarian function, host genetics, immune function and contemporary HIV and HCV treatments on liver disease progression.
Keywords: HIV & AIDS; hepatology; infectious diseases.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AA has received consulting fees from Merck, Viiv Healthcare and Gilead Sciences; Merck and Gilead Sciences have provided her institution with funding for her research. JCP has received consulting fees from Theratechnologies; Gilead Sciences and Merck have provided her institution with funding for her research. PCT: Merck has provided her institution with funding for her research; Gilead and Lilly have also provided her institution with funding for her to conduct industry-sponsored clinical trials. MHK reports consulting for Sanofi. All other authors have no conflicts of interest to report.
Figures
References
-
- World Health Organization . Global health Observatory (GHO) data: number of women living with HIV. Geneva: World Health Organization, 2017.
-
- Centers for Disease Control and Prevention . Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report 2019;24(No. 1), 2019. Available: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html [Accessed 31 Oct 2019].
Publication types
MeSH terms
Grants and funding
- U01 AI031834/AI/NIAID NIH HHS/United States
- U01 HL146208/HL/NHLBI NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- U01 HL146192/HL/NHLBI NIH HHS/United States
- U01 HL146242/HL/NHLBI NIH HHS/United States
- U01 HL146193/HL/NHLBI NIH HHS/United States
- R01 DK109823/DK/NIDDK NIH HHS/United States
- U01 HL146194/HL/NHLBI NIH HHS/United States
- U01 HL146241/HL/NHLBI NIH HHS/United States
- P30 AI027767/AI/NIAID NIH HHS/United States
- P30 AI050409/AI/NIAID NIH HHS/United States
- U01 HL146333/HL/NHLBI NIH HHS/United States
- U01 HL146245/HL/NHLBI NIH HHS/United States
- K24 AI108516/AI/NIAID NIH HHS/United States
- U01 HL146205/HL/NHLBI NIH HHS/United States
- P30 MH116867/MH/NIMH NIH HHS/United States
- P30 AI073961/AI/NIAID NIH HHS/United States
- U01 HL146201/HL/NHLBI NIH HHS/United States
- U01 HL146204/HL/NHLBI NIH HHS/United States
- U01 HL146202/HL/NHLBI NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- U01 HL146240/HL/NHLBI NIH HHS/United States
- U01 HL146203/HL/NHLBI NIH HHS/United States
- UL1 TR003098/TR/NCATS NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
