Genome-wide association analyses of symptom severity among clozapine-treated patients with schizophrenia spectrum disorders
- PMID: 35393395
- PMCID: PMC8989876
- DOI: 10.1038/s41398-022-01884-3
Genome-wide association analyses of symptom severity among clozapine-treated patients with schizophrenia spectrum disorders
Abstract
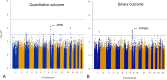
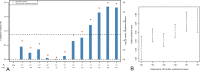
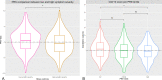
Clozapine is the most effective antipsychotic for patients with treatment-resistant schizophrenia. However, response is highly variable and possible genetic underpinnings of this variability remain unknown. Here, we performed polygenic risk score (PRS) analyses to estimate the amount of variance in symptom severity among clozapine-treated patients explained by PRSs (R2) and examined the association between symptom severity and genotype-predicted CYP1A2, CYP2D6, and CYP2C19 enzyme activity. Genome-wide association (GWA) analyses were performed to explore loci associated with symptom severity. A multicenter cohort of 804 patients (after quality control N = 684) with schizophrenia spectrum disorder treated with clozapine were cross-sectionally assessed using the Positive and Negative Syndrome Scale and/or the Clinical Global Impression-Severity (CGI-S) scale. GWA and PRS regression analyses were conducted. Genotype-predicted CYP1A2, CYP2D6, and CYP2C19 enzyme activities were calculated. Schizophrenia-PRS was most significantly and positively associated with low symptom severity (p = 1.03 × 10-3; R2 = 1.85). Cross-disorder-PRS was also positively associated with lower CGI-S score (p = 0.01; R2 = 0.81). Compared to the lowest tertile, patients in the highest schizophrenia-PRS tertile had 1.94 times (p = 6.84×10-4) increased probability of low symptom severity. Higher genotype-predicted CYP2C19 enzyme activity was independently associated with lower symptom severity (p = 8.44×10-3). While no locus surpassed the genome-wide significance threshold, rs1923778 within NFIB showed a suggestive association (p = 3.78×10-7) with symptom severity. We show that high schizophrenia-PRS and genotype-predicted CYP2C19 enzyme activity are independently associated with lower symptom severity among individuals treated with clozapine. Our findings open avenues for future pharmacogenomic projects investigating the potential of PRS and genotype-predicted CYP-activity in schizophrenia.
© 2022. The Author(s).
Conflict of interest statement
Dr. Bousman reports he has received in-kind testing kits from Myriad Neuroscience, CNSDose, Genomind, and AB-Biotics for research purposes but has not received payments or received any equity, stocks, or options in these companies or any other pharmacogenetic companies. Dr. Lähteenvuo reports being a shareholder and board member at Genomi Solutions Ltd and Nursie Health Ltd; receiving research grants or awards from the Finnish Medical Foundation and Emil Aaltonen Foundation; receiving travel grants or speakers’ honoraria from Sunovion Ltd, Janssen-Cilag, Otsuka Pharmaceutical Ltd, Lundbeck Ltd, Orion Pharma ltd; and working as a coordinator for a research project funded by the Stanley Foundation. Dr. A.E. Anil Yağcıoğlu has recently received travel grants or speakers’ honoraria from Janssen, Otsuka; is a member of the advisory board for Janssen, Otsuka; is currently participating in an international clinical trial funded by Janssen. Dr. Tiihonen reports personal fees from the Finnish Medicines Agency (Fimea), European Medicines Agency (EMA), Eli Lilly, Janssen, Lundbeck, and Otsuka; is a member of the advisory board for Lundbeck; and has participated in research projects funded by grants from Eli Lilly and Janssen to his employing institution. All other authors have declared that there are no conflicts of interest in relation to the subject of this study.
Figures

References
-
- Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: A review. Br Med Bull. 2015;114:169–79. - PubMed
-
- Kane JM, Leucht S, Carpenter D, Docherty JP. Expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: methods, commentary, and summary. J Clin Psychiatry. 2003;64:5–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

