Inhibition of BKCa channels protects neonatal hearts against myocardial ischemia and reperfusion injury
- PMID: 35393410
- PMCID: PMC8989942
- DOI: 10.1038/s41420-022-00980-z
Inhibition of BKCa channels protects neonatal hearts against myocardial ischemia and reperfusion injury
Abstract
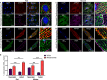
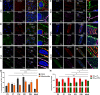
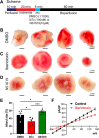
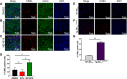
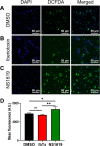
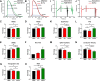
BKCa channels are large-conductance calcium and voltage-activated potassium channels that are heterogeneously expressed in a wide array of cells. Activation of BKCa channels present in mitochondria of adult ventricular cardiomyocytes is implicated in cardioprotection against ischemia-reperfusion (IR) injury. However, the BKCa channel's activity has never been detected in the plasma membrane of adult ventricular cardiomyocytes. In this study, we report the presence of the BKCa channel in the plasma membrane and mitochondria of neonatal murine and rodent cardiomyocytes, which protects the heart on inhibition but not activation. Furthermore, K+ currents measured in neonatal cardiomyocyte (NCM) was sensitive to iberiotoxin (IbTx), suggesting the presence of BKCa channels in the plasma membrane. Neonatal hearts subjected to IR when post-conditioned with NS1619 during reoxygenation increased the myocardial infarction whereas IbTx reduced the infarct size. In agreement, isolated NCM also presented increased apoptosis on treatment with NS1619 during hypoxia and reoxygenation, whereas IbTx reduced TUNEL-positive cells. In NCMs, activation of BKCa channels increased the intracellular reactive oxygen species post HR injury. Electrophysiological characterization of NCMs indicated that NS1619 increased the beat period, field, and action potential duration, and decreased the conduction velocity and spike amplitude. In contrast, IbTx had no impact on the electrophysiological properties of NCMs. Taken together, our data established that inhibition of plasma membrane BKCa channels in the NCM protects neonatal heart/cardiomyocytes from IR injury. Furthermore, the functional disparity observed towards the cardioprotective activity of BKCa channels in adults compared to neonatal heart could be attributed to their differential localization.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle.Br J Pharmacol. 1996 Jan;117(1):119-29. doi: 10.1111/j.1476-5381.1996.tb15163.x. Br J Pharmacol. 1996. PMID: 8825352 Free PMC article.
-
Expression and Activation of BKCa Channels in Mice Protects Against Ischemia-Reperfusion Injury of Isolated Hearts by Modulating Mitochondrial Function.Front Cardiovasc Med. 2019 Jan 28;5:194. doi: 10.3389/fcvm.2018.00194. eCollection 2018. Front Cardiovasc Med. 2019. PMID: 30746365 Free PMC article.
-
Activation of BKca channels mediates hippocampal neuronal death after reoxygenation and reperfusion.Mol Neurobiol. 2013 Dec;48(3):794-807. doi: 10.1007/s12035-013-8467-x. Epub 2013 May 8. Mol Neurobiol. 2013. PMID: 23653329
-
BKCa Channels as Targets for Cardioprotection.Antioxidants (Basel). 2020 Aug 17;9(8):760. doi: 10.3390/antiox9080760. Antioxidants (Basel). 2020. PMID: 32824463 Free PMC article. Review.
-
Ca(2+)-activated K(+) channels as therapeutic targets for myocardial and vascular protection.Circ J. 2015;79(3):455-62. doi: 10.1253/circj.CJ-15-0015. Epub 2015 Jan 30. Circ J. 2015. PMID: 25746520 Review.
Cited by
-
Functional large-conductance calcium and voltage-gated potassium channels in extracellular vesicles act as gatekeepers of structural and functional integrity.Nat Commun. 2025 Jan 2;16(1):42. doi: 10.1038/s41467-024-55379-4. Nat Commun. 2025. PMID: 39747826 Free PMC article.
-
Regulation of Papillary Muscle Contractility by NAD and Ammonia Interplay: Contribution of Ion Channels and Exchangers.Membranes (Basel). 2022 Dec 7;12(12):1239. doi: 10.3390/membranes12121239. Membranes (Basel). 2022. PMID: 36557146 Free PMC article.
-
Nitric oxide is involved in the cardioprotection of neonatal rat hearts, but not in neonatal ischemic postconditioning.Physiol Rep. 2024 Aug;12(15):e16147. doi: 10.14814/phy2.16147. Physiol Rep. 2024. PMID: 39097984 Free PMC article.
-
The BKCa (slo) channel regulates the cardiac function of Drosophila.Physiol Rep. 2024 Apr;12(7):e15996. doi: 10.14814/phy2.15996. Physiol Rep. 2024. PMID: 38561252 Free PMC article.
References
Grants and funding
- R01 HL133050/HL/NHLBI NIH HHS/United States
- HL157453/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL157453/HL/NHLBI NIH HHS/United States
- HL133050/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- 20CDA35310714/American Heart Association (American Heart Association, Inc.)
LinkOut - more resources
Full Text Sources

