Skeletal muscle differentiation of human iPSCs meets bioengineering strategies: perspectives and challenges
- PMID: 35393412
- PMCID: PMC8991236
- DOI: 10.1038/s41536-022-00216-9
Skeletal muscle differentiation of human iPSCs meets bioengineering strategies: perspectives and challenges
Abstract
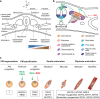
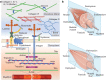
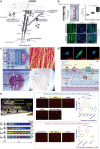
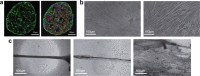
Although skeletal muscle repairs itself following small injuries, genetic diseases or severe damages may hamper its ability to do so. Induced pluripotent stem cells (iPSCs) can generate myogenic progenitors, but their use in combination with bioengineering strategies to modulate their phenotype has not been sufficiently investigated. This review highlights the potential of this combination aimed at pushing the boundaries of skeletal muscle tissue engineering. First, the overall organization and the key steps in the myogenic process occurring in vivo are described. Second, transgenic and non-transgenic approaches for the myogenic induction of human iPSCs are compared. Third, technologies to provide cells with biophysical stimuli, biomaterial cues, and biofabrication strategies are discussed in terms of recreating a biomimetic environment and thus helping to engineer a myogenic phenotype. The embryonic development process and the pro-myogenic role of the muscle-resident cell populations in co-cultures are also described, highlighting the possible clinical applications of iPSCs in the skeletal muscle tissue engineering field.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Grogan BF, Hsu MAJJR. Volumetric Muscle Loss. J. Am. Acad. Orthop. Surg. 2011;19:35–37. - PubMed
-
- Osaki T, Uzel SGM, Kamm RD. On-chip 3D neuromuscular model for drug screening and precision medicine in neuromuscular disease. Nat. Protoc. 2020;15:421–449. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem. Cells Mouse Embryonic Adult Fibroblast Cult. Defined Factors Cell. 2006;126:663–676. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

