SorCS3 promotes the internalization of p75NTR to inhibit GBM progression
- PMID: 35393432
- PMCID: PMC8989992
- DOI: 10.1038/s41419-022-04753-5
SorCS3 promotes the internalization of p75NTR to inhibit GBM progression
Abstract
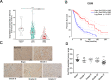
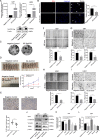
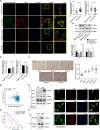
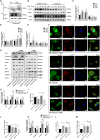
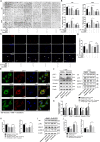
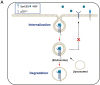
Glioblastoma (GBM) is a fatal malignancy caused by dysregulation of cellular signal transduction. Internalization plays a key role in maintaining signalling balance. Previous reports showed that Sortilin related VPS10 domain containing receptor 3 (SorCS3) has the ability to regulate internalization. However, the impacts of SorCS3 on the biological processes involved in GBM have not yet been reported. In this study, we investigated the bio-function of SorCS3 in GBM. We found that SorCS3 was significantly downregulated in GBM. In addition, low expression level of SorCS3 predicted poor prognoses in patients with GBM. Here, we proved that SorCS3 suppressed cell invasion and proliferation mainly via NGF/p75NTR pathway in GBM. We found that SorCS3 co-localized with p75NTR in GBM cells and regulated the p75NTR protein level by promoting trafficking of the endosomal to the lysosome. Immunofluorescence (IF) and Co-Immunoprecipitation (Co-IP) detection confirmed that SorCS3 bound to p75NTR, which subsequently increased the internalization of p75NTR, and then transported p75NTR to the lysosome for degradation, ultimately contributing to inhibit of glioma progression. Taken together, our work suggests that SorCS3 is a marker of promising prognosis in GBM patients and suggests that SorCS3 regulates internalization, which plays a pivotal role in inhibiting glioma progression.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Glioma invasion mediated by the p75 neurotrophin receptor (p75(NTR)/CD271) requires regulated interaction with PDLIM1.Oncogene. 2016 Mar 17;35(11):1411-22. doi: 10.1038/onc.2015.199. Epub 2015 Jun 29. Oncogene. 2016. PMID: 26119933 Free PMC article.
-
The Role of Neurotrophin Signaling in Gliomagenesis: A Focus on the p75 Neurotrophin Receptor (p75NTR/CD271).Vitam Horm. 2017;104:367-404. doi: 10.1016/bs.vh.2016.11.001. Epub 2017 Jan 4. Vitam Horm. 2017. PMID: 28215302 Review.
-
The p75 neurotrophin receptor enhances TrkA signalling by binding to Shc and augmenting its phosphorylation.J Neurochem. 2004 Apr;89(2):344-53. doi: 10.1111/j.1471-4159.2004.02344.x. J Neurochem. 2004. PMID: 15056278
-
Mapping of the interaction site between sortilin and the p75 neurotrophin receptor reveals a regulatory role for the sortilin intracellular domain in p75 neurotrophin receptor shedding and apoptosis.J Biol Chem. 2012 Dec 21;287(52):43798-809. doi: 10.1074/jbc.M112.374710. Epub 2012 Oct 26. J Biol Chem. 2012. PMID: 23105113 Free PMC article.
-
Role of p75 neurotrophin receptor in stem cell biology: more than just a marker.Cell Mol Life Sci. 2014 Jul;71(13):2467-81. doi: 10.1007/s00018-014-1564-9. Epub 2014 Jan 31. Cell Mol Life Sci. 2014. PMID: 24481864 Free PMC article. Review.
Cited by
-
Multi-omics approach reveals dysregulated genes during hESCs neuronal differentiation exposure to paracetamol.iScience. 2023 Aug 28;26(10):107755. doi: 10.1016/j.isci.2023.107755. eCollection 2023 Oct 20. iScience. 2023. PMID: 37731623 Free PMC article.
-
A triple serine motif in the intracellular domain of SorCS2 impacts its cellular signaling.iScience. 2025 May 19;28(6):112695. doi: 10.1016/j.isci.2025.112695. eCollection 2025 Jun 20. iScience. 2025. PMID: 40520096 Free PMC article.
-
Implicating the cholecystokinin B receptor in liver stem cell oncogenesis.Am J Physiol Gastrointest Liver Physiol. 2024 Mar 1;326(3):G291-G309. doi: 10.1152/ajpgi.00208.2023. Epub 2024 Jan 22. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38252699 Free PMC article.
-
Finding memo: versatile interactions of the VPS10p-Domain receptors in Alzheimer's disease.Mol Neurodegener. 2022 Nov 18;17(1):74. doi: 10.1186/s13024-022-00576-2. Mol Neurodegener. 2022. PMID: 36397124 Free PMC article.
-
ERCC1/NGFR affects prognosis in basal-like breast cancer.Eur J Med Res. 2025 Jul 9;30(1):597. doi: 10.1186/s40001-025-02807-w. Eur J Med Res. 2025. PMID: 40629424 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

