TRAIL/S-layer/graphene quantum dot nanohybrid enhanced stability and anticancer activity of TRAIL on colon cancer cells
- PMID: 35393438
- PMCID: PMC8991220
- DOI: 10.1038/s41598-022-09660-5
TRAIL/S-layer/graphene quantum dot nanohybrid enhanced stability and anticancer activity of TRAIL on colon cancer cells
Abstract
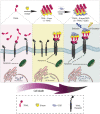
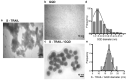
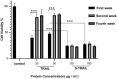
Tumor necrosis factor (TNF)-related apoptosis inducing ligand (TRAIL), known as a cytokine of the TNF superfamily, is considered a promising antitumor agent due to its ability to selectively induce apoptosis in a wide variety of cancer cells. However, failure of its successful translation into clinic has led to development of nano-based platforms aiming to improve TRAIL therapeutic efficacy. In this regard, we fabricated a novel TRAIL-S-layer fusion protein (S-TRAIL) conjugated with graphene quantum dots (GQDs) to benefit both the self-assembly of S-layer proteins, which leads to elevated TRAIL functional stability, and unique optical properties of GQDs. Noncovalent conjugation of biocompatible GQDs and soluble fusion protein was verified via UV-visible and fluorescence spectroscopy, size and ζ-potential measurements and transmission electron microscopy. The potential anticancer efficacy of the nanohybrid system on intrinsically resistant cells to TRAIL (HT-29 human colon carcinoma cells) was investigated by MTT assay and flow cytometry, which indicated about 80% apoptosis in cancer cells. These results highlight the potential of TRAIL as a therapeutic protein that can be extensively improved by taking advantage of nanotechnology and introduce S-TRAIL/GQD complex as a promising nanohybrid system in cancer treatment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
3-Bromopyruvate potentiates TRAIL-induced apoptosis in human colon cancer cells through a reactive oxygen species- and caspase-dependent mitochondrial pathway.Can J Physiol Pharmacol. 2019 Dec;97(12):1176-1184. doi: 10.1139/cjpp-2019-0131. Epub 2019 Sep 6. Can J Physiol Pharmacol. 2019. PMID: 31491344
-
The flavonolignan silibinin potentiates TRAIL-induced apoptosis in human colon adenocarcinoma and in derived TRAIL-resistant metastatic cells.Apoptosis. 2012 Aug;17(8):797-809. doi: 10.1007/s10495-012-0731-4. Apoptosis. 2012. PMID: 22555452
-
2-methoxy-5-amino-N-hydroxybenzamide sensitizes colon cancer cells to TRAIL-induced apoptosis by regulating death receptor 5 and survivin expression.Mol Cancer Ther. 2011 Oct;10(10):1969-81. doi: 10.1158/1535-7163.MCT-11-0316. Epub 2011 Aug 4. Mol Cancer Ther. 2011. PMID: 21817114
-
Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL).J Clin Oncol. 2008 Jul 20;26(21):3621-30. doi: 10.1200/JCO.2007.15.7198. J Clin Oncol. 2008. PMID: 18640940 Review.
-
Nanocarriers for TRAIL delivery: driving TRAIL back on track for cancer therapy.Nanoscale. 2017 Sep 28;9(37):13879-13904. doi: 10.1039/c7nr04959e. Nanoscale. 2017. PMID: 28914952 Review.
Cited by
-
Recent advances in targeted drug delivery systems for resistant colorectal cancer.Cancer Cell Int. 2022 May 19;22(1):196. doi: 10.1186/s12935-022-02605-y. Cancer Cell Int. 2022. PMID: 35590367 Free PMC article. Review.
-
Research Progress of Conjugated Nanomedicine for Cancer Treatment.Pharmaceutics. 2022 Jul 21;14(7):1522. doi: 10.3390/pharmaceutics14071522. Pharmaceutics. 2022. PMID: 35890416 Free PMC article. Review.
-
Engineered Graphene Quantum Dots as a Magnetic Resonance Signal Amplifier for Biomedical Imaging.Molecules. 2023 Mar 3;28(5):2363. doi: 10.3390/molecules28052363. Molecules. 2023. PMID: 36903608 Free PMC article.
-
Assessing causal associations between TNF-related apoptosis-inducing ligand, vascular endothelial growth factor and colon cancer: a Mendelian-randomization study.Discov Oncol. 2025 Jun 18;16(1):1146. doi: 10.1007/s12672-025-02864-x. Discov Oncol. 2025. PMID: 40531284 Free PMC article.
-
Bidirectional Mendelian Randomization of Causal Relationship between Inflammatory Cytokines and Different Pathological Types of Lung Cancer.J Cancer. 2024 Jul 16;15(15):4969-4984. doi: 10.7150/jca.98301. eCollection 2024. J Cancer. 2024. PMID: 39132165 Free PMC article.
References
-
- Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat. Rev. Cancer. 2005;5:876–885. - PubMed
-
- Lim SM, et al. Improved biological half-life and anti-tumor activity of TNF-related apoptosis-inducing ligand (TRAIL) using PEG-exposed nanoparticles. Biomaterials. 2011;32:3538–3546. - PubMed
-
- Byeon HJ, et al. Four-arm PEG cross-linked hyaluronic acid hydrogels containing PEGylated apoptotic TRAIL protein for treating pancreatic cancer. Acta Biomater. 2014;10:142–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

