Individual treatment effect estimation in the presence of unobserved confounding using proxies: a cohort study in stage III non-small cell lung cancer
- PMID: 35393451
- PMCID: PMC8989977
- DOI: 10.1038/s41598-022-09775-9
Individual treatment effect estimation in the presence of unobserved confounding using proxies: a cohort study in stage III non-small cell lung cancer
Abstract
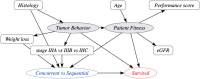
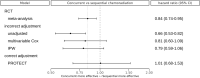
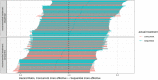
Randomized Controlled Trials (RCT) are the gold standard for estimating treatment effects but some important situations in cancer care require treatment effect estimates from observational data. We developed "Proxy based individual treatment effect modeling in cancer" (PROTECT) to estimate treatment effects from observational data when there are unobserved confounders, but proxy measurements of these confounders exist. We identified an unobserved confounder in observational cancer research: overall fitness. Proxy measurements of overall fitness exist like performance score, but the fitness as observed by the treating physician is unavailable for research. PROTECT reconstructs the distribution of the unobserved confounder based on these proxy measurements to estimate the treatment effect. PROTECT was applied to an observational cohort of 504 stage III non-small cell lung cancer (NSCLC) patients, treated with concurrent chemoradiation or sequential chemoradiation. Whereas conventional confounding adjustment methods seemed to overestimate the treatment effect, PROTECT provided credible treatment effect estimates.
© 2022. The Author(s).
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at
Figures


References
-
- FDA-NIH Biomarker Working Group, Predictive Biomarker (Food and Drug Administration (US), 2016; https://www.ncbi.nlm.nih.gov/books/NBK402283/). - PubMed
-
- Ettinger, D.S. NCCN Non-small cell lung cancer guideline, Version 1.2021 (2020) (available at https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

