Evaluation of a new transcutaneous bilirubinometer in newborn infants
- PMID: 35393482
- PMCID: PMC8989875
- DOI: 10.1038/s41598-022-09788-4
Evaluation of a new transcutaneous bilirubinometer in newborn infants
Abstract
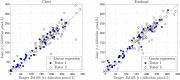
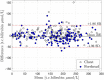
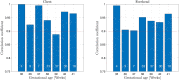
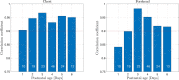
To avoid brain damage in newborn infants, effective tools for prevention of excessive neonatal hyperbilirubinemia are needed. The objective of this study was to evaluate a new transcutaneous bilirubinometer (JAISY). For this purpose, 930 bilirubin measurements were performed in 141 newborn infants born near-term or at term (gestational age 35-41 weeks; postnatal age 1-6 days; 71 boys; including 29 infants with darker skin) and compared to those of a previously validated instrument (JM105). In each infant, the mean of three repeated measurements in the forehead was calculated for each instrument, followed by a similar measurement on the chest. The bilirubin values varied between 0 and 320 µmol/l (0-18.8 mg/dl). There was a high degree of agreement with significant correlations between bilirubin values measured with the two devices on the forehead (Pearson's r = 0.94, p < 0.001) and the chest (r = 0.94, p < 0.001). The correlations remained after stratifying the data by gestational age, postnatal age and skin color. The coefficient of variation for repeated bilirubin measurements was 8.8% for JAISY and 8.0% for JM105 (p = 0.79). In conclusion, JAISY provides accurate and reproducible information on low to moderately high bilirubin levels in newborn infants born near-term or at term.
© 2022. The Author(s).
Conflict of interest statement
All three authors have shares in Jaisy Health AB, a small start-up innovation company.
Figures

Similar articles
-
Comparison of a new transcutaneous bilirubinometer (Bilimed) with serum bilirubin measurements in preterm and full-term infants.BMC Pediatr. 2009 Nov 12;9:70. doi: 10.1186/1471-2431-9-70. BMC Pediatr. 2009. PMID: 19909530 Free PMC article.
-
Predictability of transcutaneous bilirubinometry in late preterm and term infants at risk for pathological hyperbilirubinemia.J Neonatal Perinatal Med. 2021;14(2):261-267. doi: 10.3233/NPM-200486. J Neonatal Perinatal Med. 2021. PMID: 33074197
-
Comparison of transcutaneous and total serum bilirubin measurement in Turkish newborns.Turk J Pediatr. 2014 Nov-Dec;56(6):612-7. Turk J Pediatr. 2014. PMID: 26388591
-
Screening methods for neonatal hyperbilirubinemia: benefits, limitations, requirements, and novel developments.Pediatr Res. 2021 Aug;90(2):272-276. doi: 10.1038/s41390-021-01543-1. Epub 2021 May 3. Pediatr Res. 2021. PMID: 33941863 Review.
-
Jaundice and kernicterus in the moderately preterm infant.Clin Perinatol. 2013 Dec;40(4):679-88. doi: 10.1016/j.clp.2013.07.007. Epub 2013 Sep 20. Clin Perinatol. 2013. PMID: 24182955 Review.
Cited by
-
Demystifying non-invasive approaches for screening jaundice in low resource settings: a review.Front Pediatr. 2023 Nov 20;11:1292678. doi: 10.3389/fped.2023.1292678. eCollection 2023. Front Pediatr. 2023. PMID: 38054187 Free PMC article. Review.
References
-
- Alken J, Hakansson S, Ekeus C, Gustafson P, Norman M. Rates of extreme neonatal hyperbilirubinemia and kernicterus in children and adherence to national guidelines for screening, diagnosis, and treatment in Sweden. JAMA Netw. Open. 2019;2:e190858. doi: 10.1001/jamanetworkopen.2019.0858. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

