Tumour-infiltrating B cells: immunological mechanisms, clinical impact and therapeutic opportunities
- PMID: 35393541
- PMCID: PMC9678336
- DOI: 10.1038/s41568-022-00466-1
Tumour-infiltrating B cells: immunological mechanisms, clinical impact and therapeutic opportunities
Abstract
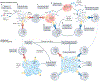
Although immunotherapy research to date has focused largely on T cells, there is mounting evidence that tumour-infiltrating B cells and plasma cells (collectively referred to as tumour-infiltrating B lymphocytes (TIL-Bs)) have a crucial, synergistic role in tumour control. In many cancers, TIL-Bs have demonstrated strong predictive and prognostic significance in the context of both standard treatments and immune checkpoint blockade, offering the prospect of new therapeutic opportunities that leverage their unique immunological properties. Drawing insights from autoimmunity, we review the molecular phenotypes, architectural contexts, antigen specificities, effector mechanisms and regulatory pathways relevant to TIL-Bs in human cancer. Although the field is young, the emerging picture is that TIL-Bs promote antitumour immunity through their unique mode of antigen presentation to T cells; their role in assembling and perpetuating immunologically 'hot' tumour microenvironments involving T cells, myeloid cells and natural killer cells; and their potential to combat immune editing and tumour heterogeneity through the easing of self-tolerance mechanisms. We end by discussing the most promising approaches to enhance TIL-B responses in concert with other immune cell subsets to extend the reach, potency and durability of cancer immunotherapy.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures




References
-
- Workel HH et al. A transcriptionally distinct CXCL13+CD103+CD8+ T-cell population is associated with B-cell recruitment and neoantigen load in human cancer. Cancer Immunol. Res 7, 784–796 (2019). - PubMed
-
- Sautès-Fridman C, Petitprez F, Calderaro J & Fridman WH Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19, 307–325 (2019). - PubMed
-
- Jhunjhunwala S, Hammer C & Delamarre L Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 21, 298–312 (2021). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

