Ionic Combisomes: A New Class of Biomimetic Vesicles to Fuse with Life
- PMID: 35393756
- PMCID: PMC9189634
- DOI: 10.1002/advs.202200617
Ionic Combisomes: A New Class of Biomimetic Vesicles to Fuse with Life
Abstract
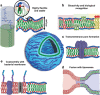
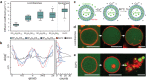
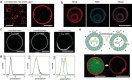
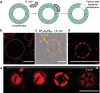
The construction of biomembranes that faithfully capture the properties and dynamic functions of cell membranes remains a challenge in the development of synthetic cells and their application. Here a new concept for synthetic cell membranes based on the self-assembly of amphiphilic comb polymers into vesicles, termed ionic combisomes (i-combisomes) is introduced. These combs consist of a polyzwitterionic backbone to which hydrophobic tails are linked by electrostatic interactions. Using a range of microscopies and molecular simulations, the self-assembly of a library of combs in water is screened. It is discovered that the hydrophobic tails form the membrane's core and force the backbone into a rod conformation with nematic-like ordering confined to the interface with water. This particular organization resulted in membranes that combine the stability of classic polymersomes with the biomimetic thickness, flexibility, and lateral mobility of liposomes. Such unparalleled matching of biophysical properties and the ability to locally reconfigure the molecular topology of its constituents enable the harboring of functional components of natural membranes and fusion with living bacteria to "hijack" their periphery. This provides an almost inexhaustible palette to design the chemical and biological makeup of the i-combisomes membrane resulting in a powerful platform for fundamental studies and technological applications.
Keywords: amphiphilic comb polymers; bottom-up synthetic biology; hybrid vesicles; polyelectrolyte-surfactant complexes; polymersomes; synthetic biomembranes; vesicle fusion.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Pepticombisomes: Biomimetic Vesicles Crafted From Recombinant Supercharged Polypeptides with Uniformly Distributed Side-Chains.Adv Sci (Weinh). 2025 Apr;12(15):e2411497. doi: 10.1002/advs.202411497. Epub 2025 Feb 22. Adv Sci (Weinh). 2025. PMID: 39985267 Free PMC article.
-
Liposomes and polymersomes: a comparative review towards cell mimicking.Chem Soc Rev. 2018 Nov 26;47(23):8572-8610. doi: 10.1039/c8cs00162f. Chem Soc Rev. 2018. PMID: 30177983 Review.
-
Poly(Sitosterol)-Based Hydrophobic Blocks in Amphiphilic Block Copolymers for the Assembly of Hybrid Vesicles.Small. 2024 Oct;20(40):e2401934. doi: 10.1002/smll.202401934. Epub 2024 Jun 11. Small. 2024. PMID: 38860565
-
Tailoring of Peptide Vesicles: A Bottom-Up Chemical Approach.Acc Chem Res. 2021 Apr 20;54(8):1934-1949. doi: 10.1021/acs.accounts.0c00690. Epub 2021 Apr 6. Acc Chem Res. 2021. PMID: 33823579
-
Polymeric vesicles: from drug carriers to nanoreactors and artificial organelles.Acc Chem Res. 2011 Oct 18;44(10):1039-49. doi: 10.1021/ar200036k. Epub 2011 May 24. Acc Chem Res. 2011. PMID: 21608994 Review.
Cited by
-
Peptide-Induced Division of Polymersomes for Biomimetic Compartmentalization.Angew Chem Int Ed Engl. 2024 Dec 20;63(52):e202413089. doi: 10.1002/anie.202413089. Epub 2024 Nov 14. Angew Chem Int Ed Engl. 2024. PMID: 39265063 Free PMC article.
-
Pepticombisomes: Biomimetic Vesicles Crafted From Recombinant Supercharged Polypeptides with Uniformly Distributed Side-Chains.Adv Sci (Weinh). 2025 Apr;12(15):e2411497. doi: 10.1002/advs.202411497. Epub 2025 Feb 22. Adv Sci (Weinh). 2025. PMID: 39985267 Free PMC article.
References
-
- a) Schwille P., Spatz J., Landfester K., Bodenschatz E., Herminghaus S., Sourjik V., Erb T. J., Bastiaens P., Lipowsky R., Hyman A., Dabrock P., Baret J. C., Vidakovic‐Koch T., Bieling P., Dimova R., Mutschler H., Robinson T., Tang T. D., Wegner S., Sundmacher K., Angew. Chem., Int. Ed. Engl. 2018, 57, 13382; - PubMed
- b) Schwille P., Angew. Chem., Int. Ed. Engl. 2017, 56, 10998; - PubMed
- c) Sato W., Zajkowski T., Moser F., Adamala K. P., Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2022, 14, e1761; - PMC - PubMed
- d) Buddingh B. C., van Hest J. C. M., Acc. Chem. Res. 2017, 50, 769; - PMC - PubMed
- e) Gopfrich K., Platzman I., Spatz J. P., Trends Biotechnol. 2018, 36, 938. - PMC - PubMed
-
- a) Luisi P. L., Anat. Rec. 2002, 268, 208; - PubMed
- b) Luisi P. L., Stano P., The minimal cell: the biophysics of cell compartment and the origin of cell functionality, Springer 2010;
- c) Rasmussen S., Stadler P., Protocells Bridging Nonliving and living matter, MIT Press, Cambridge, MA: 2009.
-
- a) Albert B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., Walter P., Molecular Biology of the Cell, 6th ed., Garland Publishing Inc, New York: 2014;
- b) Bassereau P., Jin R., Baumgart T., Deserno M., Dimova R., Frolov V. A., Bashkirov P. V., Grubmüller H., Jahn R., Risselada H. J., Johannes L., Kozlov M. M., Lipowsky R., Pucadyil T. J., Zeno W. F., Stachowiak J. C., Stamou D., Breuer A., Lauritsen L., Simon C., Sykes C., Voth G. A., Weikl T. R., J. Phys. D: Appl. Phys. 2018, 51, 343001. - PMC - PubMed
-
- a) Jacobson K., Mouritsen O. G., Anderson R. G. W., Nat. Cell Biol. 2007, 9, 7; - PubMed
- b) LoPresti C., Massignani M., Fernyhough C., Blanazs A., Ryan A. J., Madsen J., Warren N. J., Armes S. P., Lewis A. L., Chirasatitsin S., Engler A. J., Battaglia G., ACS Nano 2011, 5, 1775; - PubMed
- c) Palivan C. G., Goers R., Najer A., Zhang X., Car A., Meier W., Chem. Soc. Rev. 2016, 45, 377. - PubMed
