BNT162b2 vaccine induces antibody release in saliva: a possible role for mucosal viral protection?
- PMID: 35393790
- PMCID: PMC9081904
- DOI: 10.15252/emmm.202115326
BNT162b2 vaccine induces antibody release in saliva: a possible role for mucosal viral protection?
Abstract
Vaccination against an airborne pathogen is very effective if it induces also the development of mucosal antibodies that can protect against infection. The mRNA-based vaccine-encoding SARS-CoV-2 full-length spike protein (BNT162b2, Pfizer/BioNTech) protects also against infection despite being administered systemically. Here, we show that upon vaccination, cognate IgG molecules are also found in the saliva and are more abundant in SARS-CoV-2 previously exposed subjects, paralleling the development of plasma IgG. The antibodies titer declines at 3 months from vaccination. We identified a concentration of specific IgG in the plasma above which the relevant IgG can be detected in the saliva. Regarding IgA antibodies, we found only protease-susceptible IgA1 antibodies in plasma while they were present at very low levels in the saliva over the course of vaccination of SARS-CoV-2-naïve subjects. Thus, in response to BNT162b2 vaccine, plasma IgG can permeate into mucosal sites and participate in viral protection. It is not clear why IgA1 are detected in low amount, they may be proteolytically cleaved.
Keywords: BNT162b2; IgA; IgG; SARS-CoV-2; mucosal immunity.
© 2022 The Authors. Published under the terms of the CC BY 4.0 license.
Figures


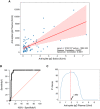
Scatterplot of the recorded values of spike SARS‐CoV‐2‐specific IgG in plasma and saliva, on the X‐axis and Y‐axis, respectively. In red, linear interpolation obtained by regression of SARS‐CoV‐2‐specific IgG in the plasma on IgG in the saliva.
ROC curve (0.9374 ± 0.02597, 95% CI 0.8865 to 0.9883 P < 0.0001).
Plot of the F1 scores computed for different threshold levels. Log values of anti‐spike IgG in the plasma are reported on X‐axis. The vertical red line highlights the maximum F1 score registered and the respective threshold value: 1.893 (log plasma) that corresponds to 78,16 IU/ml.


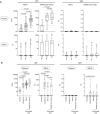
IgA1 and IgA2 subtypes specific for spike protein were measured in plasma and saliva at different time point (T0,T1, T2) of vaccinated naïve (plasma: n = 64; saliva: n = 63 (IgA1), n = 55 (IgA2)) or SARS‐CoV‐2‐Exp (plasma: n = 21; saliva: n = 18) subjects. Subjects received the first vaccine dose at day 0 (T0), and the 2nd dose at day 21 (T1) (indicated with an arrow). T2 corresponds to 7–10 days after the 2nd dose. For plasma samples, the titers of antigen‐specific IgA1 and IgA2 are expressed in IU/ml and AU, respectively (see Methods). LoD is indicated by a dotted line: LoD (IgA1) = 74.64; LoD (IgA2) = 0.009. For saliva samples, the titers of antigen‐specific Ig were normalized by dividing the values of SARS‐CoV‐2‐specific Ig by total IgA concentration of each sample. The normalization was applied only to values higher than LoD. The adjusted values are expressed in AU.
IgA1 and IgA2 subtypes specific for spike protein were measured in plasma and saliva of vaccinated naïve and SARS‐CoV‐2‐Exp subjects 7–10 days after second dose (T2), and the antibody response was compared to that obtained in COVID‐19 patients (plasma: n = 28; saliva: n = 26) and in nonvaccinated subjects (n = 19, Control).


References
-
- Abu Jabal K, Ben‐Amram H, Beiruti K, Batheesh Y, Sussan C, Zarka S, Edelstein M (2021) Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID‐19 vaccine: real‐world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill 26: 2100096 - PMC - PubMed
-
- Baeza‐Yates R, Ribeiro‐Neto B (1999) Modern information retrieval. New York, NY: ACM Press;
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

