Gene therapy of Csf2ra deficiency in mouse fetal monocyte precursors restores alveolar macrophage development and function
- PMID: 35393945
- PMCID: PMC9057586
- DOI: 10.1172/jci.insight.152271
Gene therapy of Csf2ra deficiency in mouse fetal monocyte precursors restores alveolar macrophage development and function
Abstract
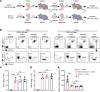
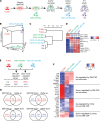
Tissue-resident macrophage-based immune therapies have been proposed for various diseases. However, generation of sufficient numbers that possess tissue-specific functions remains a major handicap. Here, we showed that fetal liver monocytes cultured with GM-CSF (CSF2-cFLiMo) rapidly differentiated into a long-lived, homogeneous alveolar macrophage-like population in vitro. CSF2-cFLiMo retained the capacity to develop into bona fide alveolar macrophages upon transfer into Csf2ra-/- neonates and prevented development of alveolar proteinosis and accumulation of apoptotic cells for at least 1 year in vivo. CSF2-cFLiMo more efficiently engrafted empty alveolar macrophage niches in the lung and protected mice from severe pathology induced by respiratory viral infection compared with transplantation of macrophages derived from BM cells cultured with M-CSF (CSF1-cBMM) in the presence or absence of GM-CSF. Harnessing the potential of this approach for gene therapy, we restored a disrupted Csf2ra gene in fetal liver monocytes and demonstrated their capacity to develop into alveolar macrophages in vivo. Altogether, we provide a platform for generation of immature alveolar macrophage-like precursors amenable for genetic manipulation, which will be useful to dissect alveolar macrophage development and function and for pulmonary transplantation therapy.
Keywords: Gene therapy; Immunology; Influenza; Macrophages.
Figures





References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

