Two-week prospective observational study of 5% sofpironium bromide gel in Japanese patients with primary axillary hyperhidrosis
- PMID: 35394087
- PMCID: PMC9321746
- DOI: 10.1111/1346-8138.16384
Two-week prospective observational study of 5% sofpironium bromide gel in Japanese patients with primary axillary hyperhidrosis
Erratum in
-
Erratum.J Dermatol. 2022 Aug;49(8):800. doi: 10.1111/1346-8138.16511. Epub 2022 Jul 12. J Dermatol. 2022. PMID: 35822282 Free PMC article. No abstract available.
Abstract
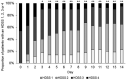
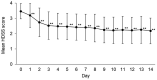
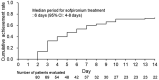
In 2020, 5% sofpironium bromide (ECCLOCK® ) gel (hereinafter referred to as sofpironium) was approved in Japan for the topical treatment of primary axillary hyperhidrosis. A phase III study of sofpironium demonstrated the efficacy and safety of sofpironium; however, no study has assessed its early efficacy at <6 weeks after starting treatment. Therefore, to assess the earlier effectiveness of sofpironium, we conducted a 2-week, single-center, exploratory, prospective, observational study in Japanese patients with primary axillary hyperhidrosis. Patients aged ≥20 years and satisfying with a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 at baseline were eligible for the study. The primary endpoint for the effectiveness was change in the proportion of patients with a HDSS score of 1, 2, 3, or 4 during the 2-week study period. In 80 patients included in the full analysis set (FAS), there were more women than men (93.8% vs. 6.3%), and the mean age (±standard deviation [SD]) was 33.3 ± 9.4 years. In the FAS, the proportion of patients with a HDSS score of 1 or 2 was 55.0% on day 7, and statistically significant changes were observed after day 3 compared to baseline (p < 0.05). Mean HDSS scores (±SD) were significantly decreased from baseline value of 3.5 ± 0.5 to 2.4 ± 0.9 on day 7 (p < 0.001). The median period for sofpironium treatment to achieve a HDSS score of 1 or 2 for a continuous 2 days was 6 days (95% confidence interval, 4-8). Safety was evaluated in 92 patients in the safety analysis set, and no adverse events were reported during the study period of 2 weeks. These results suggest that after 1-week treatment with sofpironium for patients with a HDSS score of 3 or 4, approximately 50% of the patients can achieve a HDSS score of 1 or 2, which is a clinically significant improvement for the patients.
Keywords: 2-week study; Hyperhidrosis Disease Severity Scale score; early effectiveness; primary axillary hyperhidrosis; sofpironium bromide gel.
© 2022 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
This study was funded by Kaken Pharmaceutical. T.F. received fees as a resource speaker from Kaken Pharmaceutical. H.O. and H.M. were employees of Kaken Pharmaceutical. With funding from Kaken Pharmaceutical, Medical Professional Relations assisted in the writing and editing of this paper.
Figures

Similar articles
-
A phase 3, multicenter, randomized, double-blind, vehicle-controlled, parallel-group study of 5% sofpironium bromide (BBI-4000) gel in Japanese patients with primary axillary hyperhidrosis.J Dermatol. 2021 Mar;48(3):279-288. doi: 10.1111/1346-8138.15668. Epub 2021 Jan 7. J Dermatol. 2021. PMID: 33410265 Free PMC article. Clinical Trial.
-
A phase III, 52-week, open-label study to evaluate the safety and efficacy of 5% sofpironium bromide (BBI-4000) gel in Japanese patients with primary axillary hyperhidrosis.J Dermatol. 2021 Aug;48(8):1149-1161. doi: 10.1111/1346-8138.15927. Epub 2021 May 26. J Dermatol. 2021. PMID: 34041788 Free PMC article. Clinical Trial.
-
Sofpironium topical gel, 12.45%, for the treatment of axillary hyperhidrosis: Pooled efficacy and safety results from 2 phase 3 randomized, controlled, double-blind studies.J Am Acad Dermatol. 2025 Jul;93(1):82-88. doi: 10.1016/j.jaad.2025.02.086. Epub 2025 Mar 5. J Am Acad Dermatol. 2025. PMID: 40054501 Clinical Trial.
-
Sofpironium Bromide: First Approval.Drugs. 2020 Dec;80(18):1981-1986. doi: 10.1007/s40265-020-01438-1. Drugs. 2020. PMID: 33236266 Review.
-
Efficacy and safety of topical glycopyrronium bromide in treating axillary hyperhidrosis: systematic review and meta-analysis.Sci Rep. 2024 Oct 19;14(1):24537. doi: 10.1038/s41598-024-74430-4. Sci Rep. 2024. PMID: 39424822 Free PMC article.
Cited by
-
Hyperhidrosis: A Review of Recent Advances in Treatment with Topical Anticholinergics.Dermatol Ther (Heidelb). 2022 Dec;12(12):2705-2714. doi: 10.1007/s13555-022-00838-3. Epub 2022 Nov 3. Dermatol Ther (Heidelb). 2022. PMID: 36329359 Free PMC article. Review.
-
Erratum.J Dermatol. 2022 Aug;49(8):800. doi: 10.1111/1346-8138.16511. Epub 2022 Jul 12. J Dermatol. 2022. PMID: 35822282 Free PMC article. No abstract available.
References
-
- Fujimoto T, Yokozeki H, Katayama I, Kaneda M, Murota H, Tamura N, et al. Guidelines of the Japanese dermatological association, clinical guidelines for primary focal hyperhidrosis (revised edition in 2015). Jpn J Dermatol. 2015;125:1379–400. (article in Japanese).
-
- Fujimoto T, Kawahara K, Yokozeki H. Epidemiological study and considerations of focal hyperhidrosis in Japan. From questionnaire analysis. J Dermatol. 2013;40:886–90. - PubMed
-
- Hamm H. Impact of hyperhidrosis on quality of life and its assessment. Dermatol Clin. 2014;32:467–76. - PubMed
-
- Shayesteh A, Janlert U, Nylander E. Hyperhidrosis ‐ sweating sites matter: quality of life in primary hyperhidrosis according to the sweating sites measured by SF‐36. Dermatology. 2017;233:441–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

