Neurovascular abnormalities in retinopathy of prematurity and emerging therapies
- PMID: 35394143
- PMCID: PMC9205172
- DOI: 10.1007/s00109-022-02195-2
Neurovascular abnormalities in retinopathy of prematurity and emerging therapies
Abstract
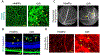
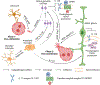
Blood vessels in the developing retina are formed in concert with neural growth, resulting in functional neurovascular network. Disruption of the neurovascular coordination contributes to the pathogenesis of retinopathy of prematurity (ROP), a potentially blinding retinal neovascular disease in preterm infants that currently lacks an approved drug therapy in the USA. Despite vasculopathy as predominant clinical manifestations, an increasing number of studies revealed complex neurovascular interplays among neurons, glial cells and blood vessels during ROP. Coordinated expression of glia-derived vascular endothelial growth factor (VEGF) in spatio-temporal gradients is pivotal to the formation of well-organized vascular plexuses in the healthy retina, whereas uncoordinated VEGF expression triggers pathological angiogenesis with disorganized vascular tufts in ROP. In contrast with VEGF driving both pathological and physiological angiogenesis, neuron-derived angiogenic factor secretogranin III (Scg3) stringently regulates ROP but not healthy retinal vessels in animal models. Anti-VEGF and anti-Scg3 therapies confer similar high efficacies to alleviate ROP in preclinical studies but are distinct in their disease selectivity and safety. This review discusses neurovascular communication among retinal blood vessels, neurons and glial cells during retinal development and ROP pathogenesis and summarizes the current and emerging therapies to address unmet clinical needs for the disease.
Keywords: Anti-angiogenic therapy; Neurovascular interaction; Oxygen-induced retinopathy; Retinopathy of prematurity; Secretogranin III; Vascular endothelial growth factor.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

