A Randomized, Controlled Comparison of NCX 470 (0.021%, 0.042%, and 0.065%) and Latanoprost 0.005% in Patients With Open-angle Glaucoma or Ocular Hypertension: The Dolomites Study
- PMID: 35394456
- PMCID: PMC9148672
- DOI: 10.1097/IJG.0000000000002030
A Randomized, Controlled Comparison of NCX 470 (0.021%, 0.042%, and 0.065%) and Latanoprost 0.005% in Patients With Open-angle Glaucoma or Ocular Hypertension: The Dolomites Study
Abstract
Prcis: NCX 470 0.042% and 0.065% were statistically superior in intraocular pressure (IOP) lowering to latanoprost 0.005%, and NCX 470 0.021% was noninferior. All NCX 470 concentrations were safe and well tolerated.
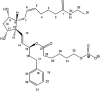
Purpose: The purpose of this study was to compare varying concentrations of NCX 470 (a nitric oxide-donating bimatoprost) to latanoprost in a dose-response safety and efficacy trial.
Patients and methods: Adult patients with bilateral open-angle glaucoma or ocular hypertension were randomized to NCX 470 0.021% (n=111), 0.042% (n=108), 0.065% (n=107), or latanoprost 0.005% (n=107) once daily in the evening. IOP was measured at 8:00 am, 10:00 am, and 4:00 pm at weeks 1, 2, and 4. The primary efficacy endpoint was the reduction from baseline in mean diurnal IOP at week 4. Secondary efficacy endpoints included reductions from baseline in mean diurnal IOP at weeks 1 and 2, and reductions from baseline in time-matched IOP at 8:00 am, 10:00 am, and 4:00 pm at weeks 1, 2, and 4. Adverse events were evaluated.
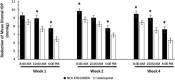
Results: All concentrations of NCX 470 resulted in significant reductions of mean diurnal IOP. The 0.042% and 0.065% concentrations were statistically superior to latanoprost 0.005%, and 0.021% was noninferior to latanoprost for change from baseline in mean diurnal IOP at week 4. The 0.065% concentration was also superior to latanoprost by up to 1.4 mm Hg for reduction from baseline at 8:00 am, 10:00 am, and 4:00 pm at week 4. NCX 470 was safe and well tolerated; conjunctival hyperemia was the most frequently reported adverse event.
Conclusions: NCX 470 demonstrated dose-dependent reductions in IOP. The 0.042% and 0.065% concentrations demonstrated significantly greater reductions from baseline in mean diurnal IOP than latanoprost 0.005% at week 4, suggesting that higher concentrations may show even greater efficacy.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Disclosure: The authors declare no conflict of interest.
Figures

References
-
- Jonas JB, Aung T, Bourne RR, et al. . Glaucoma. Lancet. 2017;309:2183–2193. - PubMed
-
- Tham YC, Li X, Wong TY, et al. . Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. - PubMed
-
- Leske MC, Heijl A, Hussein M, et al. . Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. - PubMed
-
- Noecker RS, Dirks MS, Choplin NT, et al. . A six-month randomized clinical trial comparing the intraocular pressure lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol. 2003;135:55–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

