The impact of pausing the Oxford-AstraZeneca COVID-19 vaccine on uptake in Europe: a difference-in-differences analysis
- PMID: 35394507
- PMCID: PMC9341841
- DOI: 10.1093/eurpub/ckac039
The impact of pausing the Oxford-AstraZeneca COVID-19 vaccine on uptake in Europe: a difference-in-differences analysis
Abstract
Background: Several countries paused their rollouts of the Oxford-AstraZeneca coronavirus disease-19 (COVID-19) vaccine in mid-March 2021 due to concerns about vaccine-induced thrombosis and thrombocytopenia. Many warned that this risked damaging public confidence during a critical period of pandemic response. This study investigated whether the pause in the use of the Oxford-AstraZeneca vaccine had an impact on subsequent vaccine uptake in European countries.
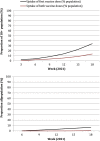
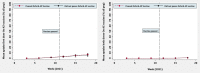
Methods: We used a difference-in-differences approach capitalizing on the fact that some countries halted their rollouts whilst others did not. A longitudinal panel was constructed for European Economic Area countries spanning 15 weeks in early 2021. Media reports were used to identify countries that paused the Oxford-AstraZeneca vaccine and the timing of this. Data on vaccine uptake were available through the European Centre for Disease Control and Prevention COVID-19 Vaccine Tracker. Difference-in-differences linear regression models controlled for key confounders that could influence vaccine uptake, and country and week fixed effects. Further models and robustness checks were performed.
Results: The panel included 28 countries, with 19 in the intervention group and 9 in the control group. Pausing the Oxford-AstraZeneca vaccine was associated with a 0.52% decrease in uptake for the first dose of a COVID-19 vaccine and a 1.49% decrease in the uptake for both doses, comparing countries that paused to those that did not. These estimates are not statistically significant (P = 0.86 and 0.39, respectively). For the Oxford-AstraZeneca vaccine only, the pause was associated with a 0.56% increase in uptake for the first dose and a 0.07% decrease in uptake for both doses. These estimates are also not statistically significant (P = 0.56 and 0.51, respectively). All our findings are robust to sensitivity analyses.
Conclusions: As new COVID-19 vaccines emerge, regulators should be cautious to deviate from usual protocols if further investigation on clinical or epidemiological grounds is warranted.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Public Health Association.
Figures

References
-
- Vogel G, Kupferschmidt K.. ‘It's a very special picture.’ Why vaccine safety experts put the brakes on AstraZeneca's COVID-19 vaccine. Science 2021. doi: 10.1126/science.abi5259. Available at https://www.science.org/content/article/it-s-very-special-picture-why-va....
-
- The Guardian. Which European States have Paused AstraZeneca Jabs Due to Clotting Concerns? 2021. Available at: https://www.theguardian.com/society/2021/mar/15/which-european-states-ha... (01 June 2021, date last accessed).
-
- Evening Standard. Finland Suspends Use of AstraZeneca Vaccine Over Blood Clot Concerns. 2021. Available at: https://www.standard.co.uk/news/world/finland-suspends-astrazeneca-vacci... (01 June 2021, date last accessed).
-
- European Medicines Agency. COVID-19 Vaccine AstraZeneca: PRAC Investigating Cases of Thromboembolic Events—Vaccine’s Benefits Currently Still Outweigh Risks—Update. 2021. Available at: https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-prac-inve... (01 June 2021, date last accessed).
-
- UN News. WHO Backs AstraZeneca COVID Vaccine Amid Clotting Concerns; Green Lights Johnson & Johnson Shots. 2021. Available at: https://news.un.org/en/story/2021/03/1087222 (01 June 2021, date last accessed).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

