Genital inflammatory status and the innate immune response to contraceptive initiation
- PMID: 35394678
- PMCID: PMC10909525
- DOI: 10.1111/aji.13542
Genital inflammatory status and the innate immune response to contraceptive initiation
Abstract
Problem: Data on the effects of contraceptives on female genital tract (FGT) immune mediators are inconsistent, possibly in part due to pre-existing conditions that influence immune mediator changes in response to contraceptive initiation.
Methods: This study included 161 South African women randomised to injectable depot medroxyprogesterone acetate (DMPA-IM), copper intrauterine device (IUD), or levonorgestrel (LNG) implant in the Evidence for Contraceptive Options and HIV Outcomes (ECHO) trial. We measured thirteen cytokines and antimicrobial peptides previously associated with HIV acquisition in vaginal swabs using Luminex and ELISA, before, and at 1 and 3 months after contraceptive initiation. Women were grouped according to an overall baseline inflammatory profile. We evaluated modification of the relationships between contraceptives and immune mediators by baseline inflammation, demographic, and clinical factors.
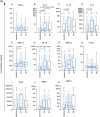
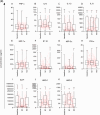
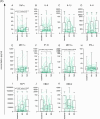
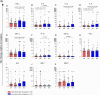
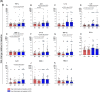
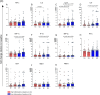
Results: Overall, LNG implant and copper IUD initiation were associated with increases in inflammatory cytokines, while no changes were observed following DMPA-IM initiation. However, when stratifying by baseline inflammatory profile, women with low baseline inflammation in all groups experienced significant increases in inflammatory cytokines, while those with a high baseline inflammatory profile experienced no change or decreases in inflammatory cytokines.
Conclusion: We conclude that pre-contraceptive initiation immune profile modifies the effect of contraceptives on the FGT innate immune response.
Keywords: contraception; copper; cytokines; female; inflammation; intrauterine devices; levonorgestrel; medroxyprogesterone acetate.
© 2022 The Authors. American Journal of Reproductive Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
There is no competing interest.
Figures
References
-
- United Nations ‐ Department of Economic and Social Affairs . Contraceptive use by Method 2019 ‐ Data Booklet. United Nations ‐ Department of Economic and Social Affairs; 2019.
-
- Polis CB, Curtis KM, Hannaford PC, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014;90:360‐390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

