Photocatalytic Aerobic Dehydrogenation of N-Heterocycles with Ir(III) Photosensitizers Bearing the 2(2'-Pyridyl)benzimidazole Scaffold
- PMID: 35394766
- PMCID: PMC9044454
- DOI: 10.1021/acs.inorgchem.2c00358
Photocatalytic Aerobic Dehydrogenation of N-Heterocycles with Ir(III) Photosensitizers Bearing the 2(2'-Pyridyl)benzimidazole Scaffold
Abstract
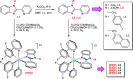
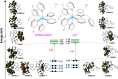
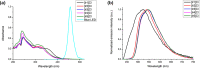
Photoredox catalysis constitutes a very powerful tool in organic synthesis, due to its versatility, efficiency, and the mild conditions required by photoinduced transformations. In this paper, we present an efficient and selective photocatalytic procedure for the aerobic oxidative dehydrogenation of partially saturated N-heterocycles to afford the respective N-heteroarenes (indoles, quinolines, acridines, and quinoxalines). The protocol involves the use of new Ir(III) biscyclometalated photocatalysts of the general formula [Ir(C^N)2(N^N')]Cl, where the C^N ligand is 2-(2,4-difluorophenyl)pyridinate, and N^N' are different ligands based on the 2-(2'-pyridyl)benzimidazole scaffold. In-depth electrochemical and photophysical studies as well as DFT calculations have allowed us to establish structure-activity relationships, which provide insights for the rational design of efficient metal-based dyes in photocatalytic oxidation reactions. In addition, we have formulated a dual mechanism, mediated by the radical anion superoxide, for the above-mentioned transformations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Rational design of mitochondria targeted thiabendazole-based Ir(III) biscyclometalated complexes for a multimodal photodynamic therapy of cancer.J Inorg Biochem. 2022 Jun;231:111790. doi: 10.1016/j.jinorgbio.2022.111790. Epub 2022 Mar 14. J Inorg Biochem. 2022. PMID: 35306449
-
Strong Influence of the Ancillary Ligand over the Photodynamic Anticancer Properties of Neutral Biscyclometalated IrIII Complexes Bearing 2-Benzoazole-Phenolates.Chemistry. 2018 Nov 27;24(66):17523-17537. doi: 10.1002/chem.201803784. Epub 2018 Nov 2. Chemistry. 2018. PMID: 30176086
-
Lanthanide Photocatalysis.Acc Chem Res. 2018 Nov 20;51(11):2926-2936. doi: 10.1021/acs.accounts.8b00336. Epub 2018 Oct 18. Acc Chem Res. 2018. PMID: 30335356
-
Radical Transformations towards the Synthesis of Quinoline: A Review.Chem Asian J. 2020 Dec 14;15(24):4153-4167. doi: 10.1002/asia.202001156. Epub 2020 Nov 16. Chem Asian J. 2020. PMID: 33135361 Review.
-
Recent advances in the synthesis of N-heteroarenes via catalytic dehydrogenation of N-heterocycles.Chem Commun (Camb). 2021 Dec 7;57(97):13042-13058. doi: 10.1039/d1cc04919d. Chem Commun (Camb). 2021. PMID: 34781335 Review.
Cited by
-
Tunable Emissive Ir(III) Benzimidazole-quinoline Hybrids as Promising Theranostic Lead Compounds.ChemMedChem. 2022 Sep 16;17(18):e202200244. doi: 10.1002/cmdc.202200244. Epub 2022 Jul 13. ChemMedChem. 2022. PMID: 35767349 Free PMC article.
-
Phenylbenzothiazole-Based Platinum(II) and Diplatinum(II) and (III) Complexes with Pyrazolate Groups: Optical Properties and Photocatalysis.Inorg Chem. 2024 Jan 22;63(3):1589-1606. doi: 10.1021/acs.inorgchem.3c03532. Epub 2024 Jan 10. Inorg Chem. 2024. PMID: 38247362 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

