Accurate Sequence-Dependent Coarse-Grained Model for Conformational and Elastic Properties of Double-Stranded DNA
- PMID: 35394775
- PMCID: PMC9097290
- DOI: 10.1021/acs.jctc.2c00138
Accurate Sequence-Dependent Coarse-Grained Model for Conformational and Elastic Properties of Double-Stranded DNA
Abstract
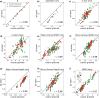
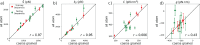
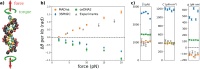
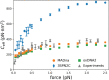
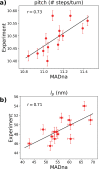
We introduce MADna, a sequence-dependent coarse-grained model of double-stranded DNA (dsDNA), where each nucleotide is described by three beads localized at the sugar, at the base moiety, and at the phosphate group, respectively. The sequence dependence is included by considering a step-dependent parametrization of the bonded interactions, which are tuned in order to reproduce the values of key observables obtained from exhaustive atomistic simulations from the literature. The predictions of the model are benchmarked against an independent set of all-atom simulations, showing that it captures with high fidelity the sequence dependence of conformational and elastic features beyond the single step considered in its formulation. A remarkably good agreement with experiments is found for both sequence-averaged and sequence-dependent conformational and elastic features, including the stretching and torsion moduli, the twist-stretch and twist-bend couplings, the persistence length, and the helical pitch. Overall, for the inspected quantities, the model has a precision comparable to atomistic simulations, hence providing a reliable coarse-grained description for the rationalization of single-molecule experiments and the study of cellular processes involving dsDNA. Owing to the simplicity of its formulation, MADna can be straightforwardly included in common simulation engines. Particularly, an implementation of the model in LAMMPS is made available on an online repository to ease its usage within the DNA research community.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

