Translational recoding by chemical modification of non-AUG start codon ribonucleotide bases
- PMID: 35394828
- PMCID: PMC11706245
- DOI: 10.1126/sciadv.abm8501
Translational recoding by chemical modification of non-AUG start codon ribonucleotide bases
Abstract
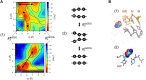
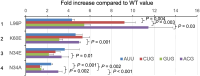
In contrast to prokaryotes wherein GUG and UUG are permissive start codons, initiation frequencies from non-AUG codons are generally low in eukaryotes, with CUG being considered as strongest. Here, we report that combined 5-cytosine methylation (5mC) and pseudouridylation (Ψ) of near-cognate non-AUG start codons convert GUG and UUG initiation strongly favored over CUG initiation in eukaryotic translation under a certain context. This prokaryotic-like preference is attributed to enhanced NUG initiation by Ψ in the second base and reduced CUG initiation by 5mC in the first base. Molecular dynamics simulation analysis of tRNAiMet anticodon base pairing to the modified codons demonstrates that Ψ universally raises the affinity of codon:anticodon pairing within the ribosomal preinitiation complex through partially mitigating discrimination against non-AUG codons imposed by eukaryotic initiation factor 1. We propose that translational control by chemical modifications of start codon bases can offer a new layer of proteome diversity regulation and therapeutic mRNA technology.
Figures

References
-
- A. G. Hinnebusch, T. E. Dever, K. Asano, in Translational Control in Biology and Medicine, M. B. Mathews, N. Sonenberg, J. W. B. Hershey, Eds. (Cold Spring Harbor Lab Press, Cold Spring Harbor, NY, 2007), pp. 225–268.
-
- Zang L., Lui K. O., von Gise A., Ma Q., Ebina W., Ptaszek L. M., Später D., Xu H., Tabebordbar M., Gorbatov R., Sena B., Nahrendorf M., Briscoe D. M., Li R. A., Wagers A. J., Rossi D. J., Pu W. T., Chien K. R., Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 31, 898–907 (2013). - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

