Mutation of SLC7A14 causes auditory neuropathy and retinitis pigmentosa mediated by lysosomal dysfunction
- PMID: 35394837
- PMCID: PMC8993119
- DOI: 10.1126/sciadv.abk0942
Mutation of SLC7A14 causes auditory neuropathy and retinitis pigmentosa mediated by lysosomal dysfunction
Abstract
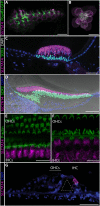
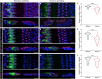
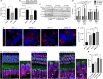
Lysosomes contribute to cellular homeostasis via processes including macromolecule degradation, nutrient sensing, and autophagy. Defective proteins related to lysosomal macromolecule catabolism are known to cause a range of lysosomal storage diseases; however, it is unclear whether mutations in proteins involved in homeostatic nutrient sensing mechanisms cause syndromic sensory disease. Here, we show that SLC7A14, a transporter protein mediating lysosomal uptake of cationic amino acids, is evolutionarily conserved in vertebrate mechanosensory hair cells and highly expressed in lysosomes of mammalian cochlear inner hair cells (IHCs) and retinal photoreceptors. Autosomal recessive mutation of SLC7A14 caused loss of IHCs and photoreceptors, leading to presynaptic auditory neuropathy and retinitis pigmentosa in mice and humans. Loss-of-function mutation altered protein trafficking and increased basal autophagy, leading to progressive cell degeneration. This study implicates autophagy-lysosomal dysfunction in syndromic hearing and vision loss in mice and humans.
Figures





References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

