The microdissected gene expression landscape of nasopharyngeal cancer reveals vulnerabilities in FGF and noncanonical NF-κB signaling
- PMID: 35394843
- PMCID: PMC8993121
- DOI: 10.1126/sciadv.abh2445
The microdissected gene expression landscape of nasopharyngeal cancer reveals vulnerabilities in FGF and noncanonical NF-κB signaling
Abstract
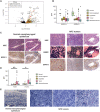
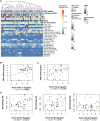
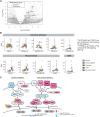
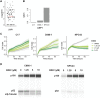
Nasopharyngeal cancer (NPC) is an Epstein-Barr virus (EBV)-positive epithelial malignancy with an extensive inflammatory infiltrate. Traditional RNA-sequencing techniques uncovered only microenvironment signatures, while the gene expression of the tumor epithelial compartment has remained a mystery. Here, we use Smart-3SEQ to prepare transcriptome-wide gene expression profiles from microdissected NPC tumors, dysplasia, and normal controls. We describe changes in biological pathways across the normal to tumor spectrum and show that fibroblast growth factor (FGF) ligands are overexpressed in NPC tumors, while negative regulators of FGF signaling, including SPRY1, SPRY2, and LGALS3, are down-regulated early in carcinogenesis. Within the NF-κB signaling pathway, the critical noncanonical transcription factors, RELB and NFKB2, are enriched in the majority of NPC tumors. We confirm the responsiveness of EBV-positive NPC cell lines to targeted inhibition of these pathways, reflecting the heterogeneity in NPC patient tumors. Our data comprehensively describe the gene expression landscape of NPC and unravel the mysteries of receptor tyrosine kinase and NF-κB pathways in NPC.
Figures


References
-
- Chang E. T., Adami H. O., The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomarkers Prev. 15, 1765–1777 (2006). - PubMed
-
- Yu M. C., Yuan J. M., Epidemiology of nasopharyngeal carcinoma. Semin. Cancer Biol. 12, 421–429 (2002). - PubMed
-
- Lanier A., Bender T., Talbot M., Wilmeth S., Tschopp C., Henle W., Henle G., Ritter D., Terasaki P., Nasopharyngeal carcinoma in Alaskan Eskimos Indians, and Aleuts: A review of cases and study of Epstein-Barr virus, HLA, and environmental risk factors. Cancer 46, 2100–2106 (1980). - PubMed
-
- Chien Y. C., Chen J. Y., Liu M. Y., Yang H. I., Hsu M. M., Chen C. J., Yang C. S., Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N. Engl. J. Med. 345, 1877–1882 (2001). - PubMed
-
- Tay J. K., Siow C. H., Goh H. L., Lim C. M., Hsu P. P., Chan S. H., A comparison of EBV serology and serum cell-free DNA as screening tools for nasopharyngeal cancer: Results of the Singapore NPC screening cohort. Int. J. Cancer 146, 2923–2931 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

