Aster proteins mediate carotenoid transport in mammalian cells
- PMID: 35394870
- PMCID: PMC9169810
- DOI: 10.1073/pnas.2200068119
Aster proteins mediate carotenoid transport in mammalian cells
Abstract
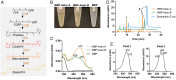
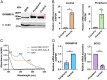
Some mammalian tissues uniquely concentrate carotenoids, but the underlying biochemical mechanism for this accumulation has not been fully elucidated. For instance, the central retina of the primate eyes displays high levels of the carotenoids, lutein, and zeaxanthin, whereas the pigments are largely absent in rodent retinas. We previously identified the scavenger receptor class B type 1 and the enzyme β-carotene-oxygenase-2 (BCO2) as key components that determine carotenoid concentration in tissues. We now provide evidence that Aster (GRAM-domain-containing) proteins, recently recognized for their role in nonvesicular cholesterol transport, engage in carotenoid metabolism. Our analyses revealed that the StART-like lipid binding domain of Aster proteins can accommodate the bulky pigments and bind them with high affinity. We further showed that carotenoids and cholesterol compete for the same binding site. We established a bacterial test system to demonstrate that the StART-like domains of mouse and human Aster proteins can extract carotenoids from biological membranes. Mice deficient for the carotenoid catabolizing enzyme BCO2 concentrated carotenoids in Aster-B protein-expressing tissues such as the adrenal glands. Remarkably, Aster-B was expressed in the human but not in the mouse retina. Within the retina, Aster-B and BCO2 showed opposite expression patterns in central versus peripheral parts. Together, our study unravels the biochemical basis for intracellular carotenoid transport and implicates Aster-B in the pathway for macula pigment concentration in the human retina.
Keywords: BCO2; GRAMD1; carotenoids; cholesterol; retina.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- von Lintig J., Colors with functions: Elucidating the biochemical and molecular basis of carotenoid metabolism. Annu. Rev. Nutr. 30, 35–56 (2010). - PubMed
-
- Álvarez R., Vaz B., Gronemeyer H., de Lera A. R., Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem. Rev. 114, 1–125 (2014). - PubMed
-
- Demmig-Adams B., W. W. Adams, 3rd, Antioxidants in photosynthesis and human nutrition. Science 298, 2149–2153 (2002). - PubMed
-
- Toews D. P. L., Hofmeister N. R., Taylor S. A., The evolution and genetics of carotenoid processing in animals. Trends Genet. 33, 171–182 (2017). - PubMed
-
- Bone R. A., Landrum J. T., Tarsis S. L., Preliminary identification of the human macular pigment. Vision Res. 25, 1531–1535 (1985). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

