Discovery of novel benzophenone integrated derivatives as anti-Alzheimer's agents targeting presenilin-1 and presenilin-2 inhibition: A computational approach
- PMID: 35395008
- PMCID: PMC8993008
- DOI: 10.1371/journal.pone.0265022
Discovery of novel benzophenone integrated derivatives as anti-Alzheimer's agents targeting presenilin-1 and presenilin-2 inhibition: A computational approach
Abstract
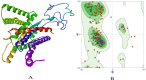
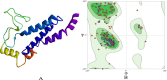
The most commonly accepted hypothesis of Alzheimer's disease (AD) is the amyloid hypothesis caused due to formation of accumulation of Aβ42 isoform, which leads to neurodegeneration. In this regard, presenilin-1 (PSEN-1) and -2 (PSEN-2) proteins play a crucial role by altering the amyloid precursor protein (APP) metabolism, affecting γ-secretase protease secretion, finally leading to the increased levels of Aβ. In the absence of reported commercial pharmacotherapeutic agents targeting presenilins, we aim to propose benzophenone integrated derivatives (BIDs) as the potential inhibitors of presenilin proteins through in silico approach. The study evaluates the interaction of BIDs through molecular docking simulations, molecular dynamics simulations, and binding free energy calculations. This is the first ever computational approach to discover the potential inhibitors of presenilin proteins. It also comprises druglikeliness and pharmacotherapeutic potential analysis of the compounds. Out of all the screened BIDs, BID-16 was found to be the lead compound against both the presenilin proteins. Based on these results, one can evaluate BID-16 as an anti-Alzheimer's potential specifically targeting presenilin proteins in near future using in vitro and in vivo methods.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


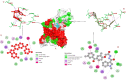
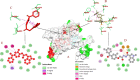
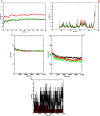
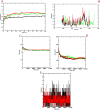
Similar articles
-
Presenilin Is Essential for ApoE Secretion, a Novel Role of Presenilin Involved in Alzheimer's Disease Pathogenesis.J Neurosci. 2022 Feb 23;42(8):1574-1586. doi: 10.1523/JNEUROSCI.2039-21.2021. Epub 2022 Jan 5. J Neurosci. 2022. PMID: 34987110 Free PMC article.
-
Analysis of Non-Amyloidogenic Mutations in APP Supports Loss of Function Hypothesis of Alzheimer's Disease.Int J Mol Sci. 2023 Jan 20;24(3):2092. doi: 10.3390/ijms24032092. Int J Mol Sci. 2023. PMID: 36768421 Free PMC article.
-
Human Presenilin-1 delivered by AAV9 rescues impaired γ-secretase activity, memory deficits, and neurodegeneration in Psen mutant mice.Proc Natl Acad Sci U S A. 2023 Oct 17;120(42):e2306714120. doi: 10.1073/pnas.2306714120. Epub 2023 Oct 10. Proc Natl Acad Sci U S A. 2023. PMID: 37816062 Free PMC article.
-
Genes and mechanisms involved in beta-amyloid generation and Alzheimer's disease.Eur Arch Psychiatry Clin Neurosci. 1999;249(6):266-70. doi: 10.1007/s004060050098. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10653281 Review.
-
γ-Secretase and its modulators: Twenty years and beyond.Neurosci Lett. 2019 May 14;701:162-169. doi: 10.1016/j.neulet.2019.02.011. Epub 2019 Feb 11. Neurosci Lett. 2019. PMID: 30763650 Free PMC article. Review.
Cited by
-
Quinoline- and Isoindoline-Integrated Polycyclic Compounds as Antioxidant, and Antidiabetic Agents Targeting the Dual Inhibition of α-Glycosidase and α-Amylase Enzymes.Pharmaceuticals (Basel). 2023 Aug 30;16(9):1222. doi: 10.3390/ph16091222. Pharmaceuticals (Basel). 2023. PMID: 37765030 Free PMC article.
-
Computer-Aided Screening of Phytoconstituents from Ocimum tenuiflorum against Diabetes Mellitus Targeting DPP4 Inhibition: A Combination of Molecular Docking, Molecular Dynamics, and Pharmacokinetics Approaches.Molecules. 2022 Aug 12;27(16):5133. doi: 10.3390/molecules27165133. Molecules. 2022. PMID: 36014373 Free PMC article.
-
Computational exploration of Picrasma quassioides compounds as CviR-mediated quorum sensing inhibitors against Chromobacterium violaceum.Front Chem. 2024 May 28;12:1286675. doi: 10.3389/fchem.2024.1286675. eCollection 2024. Front Chem. 2024. PMID: 38867763 Free PMC article.
-
Synthesis, molecular docking, analgesic, anti-inflammatory, and ulcerogenic evaluation of thiophene-pyrazole candidates as COX, 5-LOX, and TNF-α inhibitors.Inflammopharmacology. 2024 Feb;32(1):693-713. doi: 10.1007/s10787-023-01364-0. Epub 2023 Nov 20. Inflammopharmacology. 2024. PMID: 37985602
-
Screening for potential novel probiotic Levilactobacillus brevis RAMULAB52 with antihyperglycemic property from fermented Carica papaya L.Front Microbiol. 2023 Jun 20;14:1168102. doi: 10.3389/fmicb.2023.1168102. eCollection 2023. Front Microbiol. 2023. PMID: 37408641 Free PMC article.
References
-
- Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement 12: 719–732.Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(8):a006239. Published 2012 Aug 1. doi: 10.1101/cshperspect.a006239 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

