Identification of a two-component regulatory system involved in antimicrobial peptide resistance in Streptococcus pneumoniae
- PMID: 35395062
- PMCID: PMC9020739
- DOI: 10.1371/journal.ppat.1010458
Identification of a two-component regulatory system involved in antimicrobial peptide resistance in Streptococcus pneumoniae
Abstract
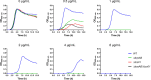
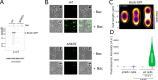
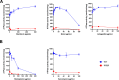
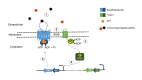
Two-component regulatory systems (TCS) are among the most widespread mechanisms that bacteria use to sense and respond to environmental changes. In the human pathogen Streptococcus pneumoniae, a total of 13 TCS have been identified and many of them have been linked to pathogenicity. Notably, TCS01 strongly contributes to pneumococcal virulence in several infection models. However, it remains one of the least studied TCS in pneumococci and its functional role is still unclear. In this study, we demonstrate that TCS01 cooperates with a BceAB-type ABC transporter to sense and induce resistance to structurally-unrelated antimicrobial peptides of bacterial origin that all target undecaprenyl-pyrophosphate or lipid II, which are essential precursors of cell wall biosynthesis. Even though tcs01 and bceAB genes do not locate in the same gene cluster, disruption of either of them equally sensitized the bacterium to the same set of antimicrobial peptides. We show that the key function of TCS01 is to upregulate the expression of the transporter, while the latter appears the main actor in resistance. Electrophoretic mobility shift assays further demonstrated that the response regulator of TCS01 binds to the promoter region of the bceAB genes, implying a direct control of these genes. The BceAB transporter was overexpressed and purified from E. coli. After reconstitution in liposomes, it displayed substantial ATPase and GTPase activities that were stimulated by antimicrobial peptides to which it confers resistance to, revealing new functional features of a BceAB-type transporter. Altogether, this inducible defense mechanism likely contributes to the survival of the opportunistic microorganism in the human host, in which competition among commensal microorganisms is a key determinant for effective host colonization and invasive path.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6(7):e744–e57. doi: 10.1016/S2214-109X(18)30247-X ; PubMed Central PMCID: PMC6005122. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

