Clinical Biology of the Pituitary Adenoma
- PMID: 35395078
- PMCID: PMC9695123
- DOI: 10.1210/endrev/bnac010
Clinical Biology of the Pituitary Adenoma
Abstract
All endocrine glands are susceptible to neoplastic growth, yet the health consequences of these neoplasms differ between endocrine tissues. Pituitary neoplasms are highly prevalent and overwhelmingly benign, exhibiting a spectrum of diverse behaviors and impact on health. To understand the clinical biology of these common yet often innocuous neoplasms, we review pituitary physiology and adenoma epidemiology, pathophysiology, behavior, and clinical consequences. The anterior pituitary develops in response to a range of complex brain signals integrating with intrinsic ectodermal cell transcriptional events that together determine gland growth, cell type differentiation, and hormonal production, in turn maintaining optimal endocrine health. Pituitary adenomas occur in 10% of the population; however, the overwhelming majority remain harmless during life. Triggered by somatic or germline mutations, disease-causing adenomas manifest pathogenic mechanisms that disrupt intrapituitary signaling to promote benign cell proliferation associated with chromosomal instability. Cellular senescence acts as a mechanistic buffer protecting against malignant transformation, an extremely rare event. It is estimated that fewer than one-thousandth of all pituitary adenomas cause clinically significant disease. Adenomas variably and adversely affect morbidity and mortality depending on cell type, hormone secretory activity, and growth behavior. For most clinically apparent adenomas, multimodal therapy controlling hormone secretion and adenoma growth lead to improved quality of life and normalized mortality. The clinical biology of pituitary adenomas, and particularly their benign nature, stands in marked contrast to other tumors of the endocrine system, such as thyroid and neuroendocrine tumors.
Keywords: Cushing’s disease; acromegaly; aggressive pituitary tumor; hypothalamus; pituitary adenoma; prolactinoma.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society.
Figures



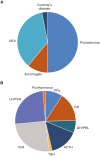
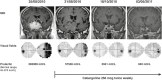
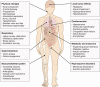
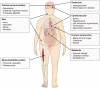
References
-
- Melmed S. Pituitary-tumor endocrinopathies. N Engl J Med. 2020;382(10):937-950. - PubMed
-
- Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA. 2017;317(5):516-524. - PubMed
-
- Yavropoulou MP, Tsoli M, Barkas K, Kaltsas G, Grossman A. The natural history and treatment of non-functioning pituitary adenomas (non-functioning PitNETs). Endocr Relat Cancer. 2020;27(10):R375-R390. - PubMed
-
- Caffarini M, Orciani M, Trementino L, Di Primio R, Arnaldi G. Pituitary adenomas, stem cells, and cancer stem cells: what’s new? J Endocrinol Invest. 2018;41(7):745-753. - PubMed

